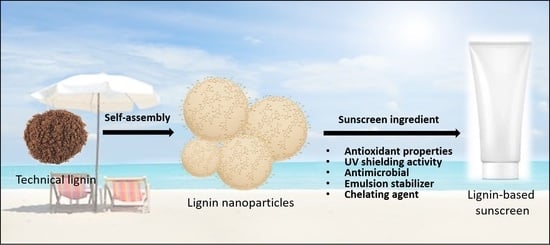
Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications
Abstract
:1. Introduction
1.1. Drawbacks of Current Antioxidant and UV Shielding Ingredients in Sunscreen Formulations
1.2. Alternative UV Shielding and Antioxidant Ingredients in Sunscreen Formulations
2. Lignin as a Novel Eco-Friendly Sunscreen Ingredient
2.1. Structure, Availability, and Green Application of Lignin
2.2. The Technology for the Self-Assembling of Lignin Nanoparticles
2.3. Antioxidant Activity
2.4. UV Shielding Activity
2.5. Other Physical and Chemical Properties of LNPs Useful in Sunscreen Formulation
2.5.1. Emulsion Stabilizer Properties
2.5.2. Antimicrobial Properties
2.5.3. Chelating Properties
3. Color Agreeableness of Lignin
3.1. Drying of Lignin
3.2. Fractionation of Lignin with Solvents
3.3. Heat Treatment
3.4. Chemical and UV Whitening of Lignin
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, P. Sun exposed skin disease. Clin. Dermatol. 2011, 29, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Sambandan, D.R.; Ratner, D. Sunscreens: An overview and update. J. Am. Acad. Dermatol. 2011, 64, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee silverskin: A review on potential cosmetic applications. Cosmetics 2018, 5, 1–5. [Google Scholar]
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Sieroń, A. Photodynamic therapy in treatment of cutaneous and choroidal melanoma. Photodiagn. Photodyn. Ther. 2013, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Karim, R.M.; Cowey, C.L. Challenging the standard of care in advanced melanoma: Focus on pembrolizumab. Cancer Manag. Res. 2017, 9, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodil, R.; Moeder, M.; Altenburger, R.; Schmitt-Jansen, M. Photostability and phytotoxicity of selected sunscreen agents and their degradation mixtures in water. Anal. Bioanal. Chem. 2009, 395, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.C. UV filters in sunscreen products—A survey. Contact Dermat. 2002, 46, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Battistin, M.; Dissette, V.; Bonetto, A.; Durini, E.; Manfredini, S.; Marcomini, A.; Casagrande, E.; Brunetta, A.; Ziosi, P.; Molesini, S.; et al. A new approach to UV protection by direct surface functionalization of TiO2 with the antioxidant polyphenol dihydroxyphenyl benzimidazole carboxylic acid. Nanomaterials 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, M. UV booster and photoprotection. In Principles and Practice of Photoprotection; Adis: Cham, Switzerland, 2016; pp. 227–245. [Google Scholar]
- Juliano, C.; Magrini, G.A. Cosmetic ingredients as emerging pollutants of environmental and health concern. A mini-review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal. Bioanal. Chem. 2012, 404, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637–638, 1279–1285. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Law, J.C.F.; Lam, T.K.; Leung, K.S.Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef]
- Nash, J.F.; Tanner, P.R. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol. Photoimmunol. Photomed. 2014, 30, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.J.M.; Miranda, M.S.; Esteves da Silva, J.C.G. The degradation products of UV filters in aqueous and chlorinated aqueous solutions. Water Res. 2012, 46, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Astolfi, P.; Giesinger, J.; Ehlis, T.; Herzog, B.; Greci, L.; Baschong, W. Assessment of the photo-degradation of UV-filters and radical-induced peroxidation in cosmetic sunscreen formulations. Free Radic. Res. 2010, 44, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, N.; Rigano, L. The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations. Cosmetics 2017, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Stein, H.V.; Berg, C.J.; Maung, J.N.; O’Connor, L.E.; Pagano, A.E.; Macmanus-Spencer, L.A.; Paulick, M.G. Photolysis and cellular toxicities of the organic ultraviolet filter chemical octyl methoxycinnamate and its photoproducts. Environ. Sci. Process. Impacts 2017, 19, 851–860. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. Antioxidants as stabilizers of UV filters: An example for the UV-B filter octylmethoxycinnamate. Biomed. Dermatology 2019, 3. [Google Scholar] [CrossRef]
- Hanson, K.M.; Gratton, E.; Bardeen, C.J. Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free Radic. Biol. Med. 2006, 41, 1205–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.B.; Casagrande, S.S.; Cowie, C.C. Urinary phenols and parabens and diabetes among US adults, NHANES 2005–2014. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Kapelewska, J.; Kotowska, U.; Wiśniewska, K. Determination of personal care products and hormones in leachate and groundwater from Polish MSW landfills by ultrasound-assisted emulsification microextraction and GC-MS. Environ. Sci. Pollut. Res. 2016, 23, 1642–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Lim, W.; Bazer, F.W.; Song, G. Avobenzone suppresses proliferative activity of human trophoblast cells and induces apoptosis mediated by mitochondrial disruption. Reprod. Toxicol. 2018, 81, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; An, S.; Lee, M.; Lee, E.; Pyo, J.J.; Kim, J.H.; Ki, M.W.; Jin, S.H.; Ha, J.; Noh, M. A long-wave UVA filter avobenzone induces obesogenic phenotypes in normal human epidermal keratinocytes and mesenchymal stem cells. Arch. Toxicol. 2019, 93, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Klopčič, I.; Dolenc, M.S. Endocrine activity of AVB, 2MR, BHA, and their mixtures. Toxicol. Sci. 2017. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Janjua, N.R.; Mogensen, B.; Andersson, A.M.; Petersen, J.H.; Henriksen, M.; Skakkebæk, N.E.; Wulf, H.C. Systemic absorption of the sunscreens benzophenone-3, octyl- methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J. Investig. Dermatol. 2004, 123, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Kunisue, T.; Chen, Z.; Buck Louis, G.M.; Sundaram, R.; Hediger, M.L.; Sun, L.; Kannan, K. Urinary concentrations of benzophenone-type UV filters in U.S. women and their association with endometriosis. Environ. Sci. Technol. 2012, 46, 4624–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiefel, C.; Schwack, W. Photoprotection in changing times—UV filter efficacy and safety, sensitization processes and regulatory aspects. Cosmet. Sci. 2014, 37, 2–30. [Google Scholar] [CrossRef] [Green Version]
- Egambaram, O.P.; Kesavan Pillai, S.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2020, 96, 779–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, J.; Londoño, C.; Ciro, Y. The health benefits of natural skin uva photoprotective compounds found in botanical sources. Int. J. Pharm. Pharm. Sci. 2016, 8, 13–23. [Google Scholar]
- Morocho-Jácome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In Vivo SPF from multifunctional sunscreen systems developed with natural compounds—A review. J. Cosmet. Dermatol. 2020. [CrossRef] [PubMed]
- Korkina, L.; Kostyuk, V.; Potapovich, A.; Mayer, W.; Talib, N.; De Luca, C. Secondary plant metabolites for sun protective cosmetics: From pre-selection to product formulation. Cosmetics 2018, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Korkina, L.G.; Mayer, W.; de Luca, C. Meristem plant cells as a sustainable source of redox actives for skin rejuvenation. Biomolecules 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takshak, S.; Agrawal, S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B Biol. 2019, 193, 51–88. [Google Scholar] [CrossRef]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient extraction and antioxidant capacity of mycosporine-like amino acids from red alga dulse palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef]
- Whittock, A.L.; Turner, M.A.P.; Coxon, D.J.L.; Woolley, J.M.; Horbury, M.D.; Stavros, V.G. Reinvestigating the Photoprotection Properties of a Mycosporine Amino Acid Motif. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiari, B.G.; Trovatti, E.; Pecoraro, É.; Corrêa, M.A.; Cicarelli, R.M.B.; Ribeiro, S.J.L.; Isaac, V.L.B. Synergistic effect of green coffee oil and synthetic sunscreen for health care application. Ind. Crops Prod. 2014, 52, 389–393. [Google Scholar] [CrossRef]
- Tomaino, A.; Cristani, M.; Cimino, F.; Speciale, A.; Trombetta, D.; Bonina, F.; Saija, A. In vitro protective effect of a Jacquez grapes wine extract on UVB-induced skin damage. Toxicol. In Vitro 2006, 20, 1395–1402. [Google Scholar] [CrossRef]
- Huynh, A.; Abou-Dahech, M.S.; Reddy, C.M.; O’Neil, G.W.; Chandler, M.; Baki, G. Alkenones, a renewably sourced, biobased wax as an SPF booster for organic sunscreens. Cosmetics 2019, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Calcio Gaudino, E.; Tabasso, S.; Grillo, G.; Cravotto, G.; Dreyer, T.; Schories, G.; Altenberg, S.; Lauberte, L.; Telysheva, G. Wheat straw lignin extraction with bio-based solvents using enabling technologies. Comptes Rendus Chem. 2018, 21, 563. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; Shupe, T.F.; Hu, T. Physicochemical characterization of lignin recovered from microwave-assisted delignified lignocellulosic biomass for use in biobased materials. J. Appl. Polym. Sci. 2015, 132, 42635–42642. [Google Scholar] [CrossRef]
- Brahim, M.; Checa Fernandez, B.L.; Regnier, O.; Boussetta, N.; Grimi, N.; Sarazin, C.; Husson, C.; Vorobiev, E.; Brosse, N. Impact of ultrasounds and high voltage electrical discharges on physico-chemical properties of rapeseed straw’s lignin and pulps. Bioresour. Technol. 2017, 237, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; Ilk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef] [Green Version]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Coll. Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Lendlein, A.; Balk, M.; Tarazona, N.A.; Oliver, E.C. Gould Bioperspectives for Shape-Memory Polymers as Shape Programmable. Act. Mater. 2019, 20, 3627–3640. [Google Scholar]
- Yuan, T.Q.; Xu, F.; Sun, R.C. Role of lignin in a biorefinery: Separation characterization and valorization. J. Chem. Technol. Biotechnol. 2013, 88, 346–352. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef] [PubMed]
- Peart, C.; Ni, Y. UV-Vis spectra of lignin model compounds in the presence of metal ions and chelants. J. Wood Chem. Technol. 2001, 21, 113–125. [Google Scholar] [CrossRef]
- Mahmood, Z.; Yameen, M.; Jahangeer, M.; Riaz, M.; Ghaffar, A.; Javid, I. Lignin as Natural Antioxidant Capacity. In Lignin—Trends and Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- García, A.; González Alriols, M.; Spigno, G.; Labidi, J. Lignin as natural radical scavenger. Effect of the obtaining and purification processes on the antioxidant behaviour of lignin. Biochem. Eng. J. 2012, 67, 173–185. [Google Scholar] [CrossRef]
- Yu, O.; Ho Kim, K. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- In Vivo Toxicity Assessment of Chitosan-Coated Lignin Nanoparticles in Embryonic Zebrafish (Danio rerio). Nanomaterials 2021, 11, 111. [CrossRef]
- Wang, B.; Sun, D.; Wang, H.M.; Yuan, T.Q.; Sun, R.C. Green and Facile Preparation of Regular Lignin Nanoparticles with High Yield and Their Natural Broad-Spectrum Sunscreens. ACS Sustain. Chem. Eng. 2019, 7, 2658–2666. [Google Scholar] [CrossRef]
- Ratanasumarn, N.; Chitprasert, P. Cosmetic potential of lignin extracts from alkaline-treated sugarcane bagasse: Optimization of extraction conditions using re-sponse surface methodology. Int. J. Biol. Macromol. 2020, 15, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Gordobil, O.; Olaizola, P.; Banales, M.J.; Labidi, J. Lignins from Agroindustrial by-Products as Natural Ingredients for Cosmetics: Chemical Structure and In Vitro Sunscreen and Cytotoxic Activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef] [Green Version]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. BioResources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H. Insights into the Potential of Hardwood Kraft Lignin to Be a Green Platform Material for Emergence of the Biorefinery. Polymers 2020, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Kirsi, S. Mikkonen. Strategies for structuring diverse emulsion systems by using wood lignocellulose-derived stabilizers. Green Chem. 2020, 22, 1019–1037. [Google Scholar]
- Kleinert, M.; Barth, T. Phenols from lignin. Chem. Eng. Technol. 2008, 31. [Google Scholar] [CrossRef]
- Martínez, Á.T.; Rencoret, J.; Marques, G.; Gutiérrez, A.; Ibarra, D.; Jiménez-Barbero, J.; del Río, J.C. Monolignol acylation and lignin structure in some nonwoody plants: A 2D NMR study. Phytochemistry 2008, 69, 2831–2843. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, G.; Hausman, J.F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Lignocellulosic biomass: Biosynthesis, degradation, and industrial utilization. Eng. Life Sci. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; Chantreau, M.; Sibout, R.; Hawkins, S. Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Davin, L.B.; Lewis, N.G. Lignin primary structures and dirigent sites. Curr. Opin. Biotechnol. 2005, 16, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Melone, F.; Sette, M.; Saladino, R. Milled wood lignin: A linear oligomer. Biomacromolecules 2011, 12, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellerstedt, G.; Henriksson, G. Monomers, Polymers and Composites from Renewable Resources; Elsevier Science: Amsterdam, The Netherlands, 2008; ISBN 9780080453163. [Google Scholar]
- Schutyser, W.; Renders, T.; Van den Bossche, G.; Van den Bosch, S.; Koelewijn, S.-F.; Ennaert, T.; Sels, B.F. Catalysis in Lignocellulosic Biorefineries: The Case of Lignin Conversion. In Nanotechnology in Catalysis; Van de Voorde, M., Sels, B., Eds.; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Wang, H.; Male, J.; Wang, Y. Recent advances in hydrotreating of pyrolysis bio-oil and its oxygen-containing model compounds. ACS Catal. 2013, 3, 1047–1070. [Google Scholar] [CrossRef]
- Giummarella, N.; Pu, Y.; Ragauskas, A.J.; Lawoko, M. A critical review on the analysis of lignin carbohydrate bonds. Green Chem. 2019, 21, 1573–1595. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M.; Hatakka, P.A.; Maciejewska, A.; Veringa, H.; Sanders, J.; Peteves, S.D.; Fernandes, E.M.; Pires, R.A.; Mano, J.F.; et al. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Carvajal-Millán, E.; Ramírez-Wong, B.; Bello-Pérez, L.A.; Montaño-Leyva, B. Ionic liquids and organic solvents for recovering lignin from lignocellulosic biomass. BioResources 2014, 9, 3660–3687. [Google Scholar] [CrossRef] [Green Version]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Ruiz-Feria, C.A.; Zhao, X. Purified lignin: Nutritional and health impacts on farm animals-A review. Anim. Feed Sci. Technol. 2008, 144, 175–184. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Eastwood, M.; Kritchevsky, D. Dietary fiber: How did we get where we are? Annu. Rev. Nutr. 2005, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nasrullah, A.; Bhat, A.H.; Isa, M.H. Lignin: A sustainable biosorbent for heavy metal adsorption from wastewater, a review. AIP Conf. Proc. 2016, 1787, 040001. [Google Scholar]
- Vinardell, M.P.; Ugartondo, V.; Mitjans, M. Potential applications of antioxidant lignins from different sources. Ind. Crops Prod. 2008, 27, 220–223. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shirono, H.; Kono, K.; Ohashi, Y. Immunopotentiating activity of the water-soluble lignin rich fraction prepared from lem—The extract of the solid culture medium of lentinus edodes mycelia. Biosci. Biotechnol. Biochem. 1997, 61, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Lignins and their derivatives with beneficial effects on human health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evstigneyev, E.I.; Shevchenko, S.M. Structure, chemical reactivity and solubility of lignin: A fresh look. Wood Sci. Technol. 2019, 53, 7–47. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Yearla, S.R.; Padmasree, K. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and UV protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Alqahtani, A.; Al-Thabit, A.; Roni, M.; Syed, R. Novel lignin nanoparticles for oral drug delivery. J. Mater. Chem. B 2019, 7, 4461–4473. [Google Scholar] [CrossRef]
- Chollet, B.; Lopez-Cuesta, J.M.; Laoutid, F.; Ferry, L. Lignin nanoparticles as a promising way for enhancing lignin flame retardant effect in polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Rallini, M.; Wang, D.Y.; Gao, D.; Dominici, F.; Torre, L.; Kenny, J.M.; Puglia, D. Role of lignin nanoparticles in UV resistance, thermal and mechanical performance of PMMA nanocomposites prepared by a combined free-radical graft polymerization/masterbatch procedure. Compos. Part A Appl. Sci. Manuf. 2018, 107, 61–69. [Google Scholar] [CrossRef]
- Rivière, G.N.; Korpi, A.; Sipponen, M.H.; Zou, T.; Kostiainen, M.A.; Österberg, M. Agglomeration of Viruses by Cationic Lignin Particles for Facilitated Water Purification. ACS Sustain. Chem. Eng. 2020, 8, 416–4177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, M.; Yuan, Q.; Cheng, G. Controlled Preparation of Corncob Lignin Nanoparticles and their Size-Dependent Antioxidant Properties: Toward High Value Utilization of Lignin. ACS Sustain. Chem. Eng. 2019, 7, 17166–17174. [Google Scholar] [CrossRef]
- Garver, T.M.; Callaghan, P.T. Hydrodynamics of Kraft Lignins. Macromolecules 1991, 24, 420–430. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, H.; Stump, A.; Ward, T.L.; Rieker, T.; Brinker, C.J. Aerosol-assisted self-assembly of mesostructured spherical nanoparticles. Nature 1999, 398, 223–226. [Google Scholar] [CrossRef]
- Borisova, O.V.; Billon, L.; Cernochova, Z.; Lapp, A.; Stepanek, P.; Borisov, O.V. Effect of temperature on self-assembly of amphiphilic block-gradient copolymers of styrene and acrylic acid. Macromol. Symp. 2015, 348, 25–32. [Google Scholar] [CrossRef]
- Guerra, A.; Gaspar, A.R.; Contreras, S.; Lucia, L.A.; Crestini, C.; Argyropoulos, D.S. On the propensity of lignin to associate: A size exclusion chromatography study with lignin derivatives isolated from different plant species. Phytochemistry 2007, 68, 2570–2583. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Feng, X.; Yang, D.; Yi, C.; Qiu, X. π-π Stacking of the aromatic groups in lignosulfonates. BioResources 2012, 7, 1145–1156. [Google Scholar]
- Zhao, W.; Xiao, L.P.; Song, G.; Sun, R.C.; He, L.; Singh, S.; Simmons, B.A.; Cheng, G. From lignin subunits to aggregates: Insights into lignin solubilization. Green Chem. 2017, 19, 3272–3281. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef] [Green Version]
- Lindström, T. The colloidal behaviour of kraft lignin. Colloid Polym. Sci. 1980, 258, 390–397. [Google Scholar] [CrossRef]
- Lindström, T.; Westman, L. The colloidal behaviour of kraft lignin. Colloid Polym. Sci. 1982, 260, 594–598. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mohanty, S.; Nayak, S.K. Synthesis, Characterization and Application of Lignin Nanoparticles (LNPs). Mater. Focus 2015, 3, 444–454. [Google Scholar] [CrossRef]
- Low, L.E.; Teh, K.C.; Siva, S.P.; Chew, I.M.L.; Mwangi, W.W.; Chew, C.L.; Goh, B.H.; Chan, E.S.; Tey, B.T. Lignin nanoparticles: The next green nanoreinforcer with wide opportunity. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100398. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A. The controlled release of bioactive compounds from lignin and lignin-based biopolymer matrices. Int. J. Biol. Macromol. 2014, 65, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Ago, M.; Huan, S.; Borghei, M.; Raula, J.; Kauppinen, E.I.; Rojas, O.J. High-throughput synthesis of lignin particles (~30 nm to ~2 μm) via aerosol flow reactor: Size fractionation and utilization in pickering emulsions. ACS Appl. Mater. Interfaces 2016, 8, 23302–23310. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Shi, Y.; Gao, B.; Wu, J.; Kirk, T.B.; Xu, J.; Xue, W. Green synthesis of lignin nanoparticle in aqueous hydrotropic solution toward broadening the window for its processing and application. Chem. Eng. J. 2018, 346, 217–225. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Ju, T.; Zhang, Z.; Li, Y.; Miao, X.; Ji, J. Continuous production of lignin nanoparticles using a microchannel reactor and its application in UV-shielding films. RSC Adv. 2019, 9, 24915–24921. [Google Scholar] [CrossRef] [Green Version]
- Beisl, S.; Friedl, A.; Miltner, A. Lignin from micro- to nanosize: Applications. Int. J. Mol. Sci. 2017, 18, 2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myint, A.A.; Lee, H.W.; Seo, B.; Son, W.S.; Yoon, J.; Yoon, T.J.; Park, H.J.; Yu, J.; Yoon, J.; Lee, Y.W. One pot synthesis of environmentally friendly lignin nanoparticles with compressed liquid carbon dioxide as an antisolvent. Green Chem. 2016, 18, 2129–2146. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Beckers, S.J.; Lu, H.; Landfester, K.; Wurm, F.R. Morphology-Controlled Synthesis of Lignin Nanocarriers for Drug Delivery and Carbon Materials. ACS Biomater. Sci. Eng. 2017, 3, 2375–2383. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and characterization of biodegradable lignin nanoparticles with tunable surface properties. Langmuir 2016, 25, 6468–6477. [Google Scholar] [CrossRef] [PubMed]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from micro- to nanosize: Production methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauce, R.; Aparecida Sales de Oliveira Pinto, C.; Robles Velasco, M.V.; Rosado, C.; Rolim Baby, A. Ex Vivo penetration analysis and anti-inflammatory efficacy of the association of ferulic acid and UV filters. Eur. J. Pharm. Sci. 2021, 156, 105578. [Google Scholar] [CrossRef]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Iqbal, A.; Shen, C.C.; Bhawani, S.A.; Adam, F. Synthesis of lignin based composites of TiO2 for potential application as radical scavengers in sunscreen formulation. BMC Chem. 2019, 13, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, F.M.C.; Cerqueira, M.A.; Gonçalves, C.; Azinheiro, S.; Garrido-Maestu, A.; Vicente, A.A.; Pastrana, L.M.; Teixeira, J.A.; Michelin, M. Green synthesis of lignin nano- and micro-particles: Physicochemical characterization, bioactive properties and cytotoxicity assessment. Int. J. Biol. Macromol. 2020, 163, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Pylypchuk, I.V.; Lindén, P.A.; Lindström, M.E.; Sevastyanova, O. New Insight into the Surface Structure of Lignin Nanoparticles Revealed by 1H Liquid-State NMR Spectroscopy. ACS Sustain. Chem. Eng. 2020, 8, 13805–13812. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Botta, L.; Bizzarri, B.M.; Bollella, P.; Antiochia, R.; Saladino, R. Layer-by-Layer Preparation of Microcapsules and Nanocapsules of Mixed Polyphenols with High Antioxidant and UV-Shielding Properties. Biomacromolecules 2018, 19, 3883–3893. [Google Scholar] [CrossRef]
- Trevisan, H.; Rezende, C.A. Pure, stable and highly antioxidant lignin nanoparticles from elephant grass. Ind. Crops Prod. 2020, 145, 112105. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X. Fabrication of uniform lignin colloidal spheres for developing natural broad-spectrum sunscreens with high sun protection factor. Ind. Crops Prod. 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Morsella, M.; D’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widsten, P.; Tamminen, T.; Liitiä, T. Natural Sunscreens Based on Nanoparticles of Modified Kraft Lignin (CatLignin). ACS Omega 2020, 5, 13438–13446. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Dai, L.; Xu, C.; Wang, K.; Zheng, C.; Si, C. Lignin-Based Micro- and Nanomaterials and their Composites in Biomedical Applications. ChemSusChem 2020, 13, 4266–4283. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light blocker-a review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Tribulová, T.; Kacík, F.; Evtuguin, D.; Cabalová, I. Assessment of chromophores in chemically treated and aged wood by uv-vis diffuse reflectance spectroscopy. Cellul. Chem. Technol. 2016, 50, 659–667. [Google Scholar]
- Wang, M.; Zhao, Y.; Li, J. From hollow lignin microsphere preparation to simultaneous preparation of urea encapsulation for controlled release using industrial kraft lignin via slow and exhaustive acetone-water evaporation. Holzforschung 2019, 74, 77–87. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, C.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Seeking Brightness from Nature: J-Aggregation-Induced Emission in Cellulolytic Enzyme Lignin Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 3169–3175. [Google Scholar] [CrossRef]
- Furman, G.S.; Lonsky, W.F.W. Charge-transfer complexes in kraft lignin part 1: Occurrence. J. Wood Chem. Technol. 1988, 8, 165–189. [Google Scholar] [CrossRef]
- Chang, H.T.; Su, Y.C.; Chang, S.T. Studies on photostability of butyrylated, milled wood lignin using spectroscopic analyses. Polym. Degrad. Stab. 2006, 91, 816–822. [Google Scholar] [CrossRef]
- Capecchi, E.; Piccinino, D.; Delfino, I.; Bollella, P.; Antiochia, R.; Saladino, R. Functionalized tyrosinase-lignin nanoparticles as sustainable catalysts for the oxidation of phenols. Nanomaterials 2018, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Piccinino, D.; Capecchi, E.; Botta, L.; Bollella, P.; Antiochia, R.; Crucianelli, M.; Saladino, R. Layer by layer supported laccase on lignin nanoparticles catalyzes the selective oxidation of alcohols to aldehydes. Catal. Sci. Technol. 2019, 9, 4125–4134. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Lu, S.; Qiu, X.; Qian, Y.; Li, P. Encapsulating TiO2 in Lignin-Based Colloidal Spheres for High Sunscreen Performance and Weak Photocatalytic Activity. ACS Sustain. Chem. Eng. 2019, 7, 6234–6242. [Google Scholar] [CrossRef]
- Yaqoob Khan, A.; Talegaonkar, S.; Iqbal, Z.; Jalees Ahmed, F.; Krishan Khar, R. Multiple Emulsions: An Overview. Curr. Drug Deliv. 2006, 3, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Benvegnu, T.; Plusquellec, D.; Lemiègre, L. Surfactants from renewable sources: Synthesis and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 15–178. [Google Scholar]
- Ruwoldt, J. A Critical Review of the Physicochemical Properties of Lignosulfonates: Chemical Structure and Behavior in Aqueous Solution, at Surfaces and Interfaces. Surfaces 2020, 3, 42. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Österberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef] [Green Version]
- Oh-Hara, T.; Sakagami, H.; Kawazoe, Y.; Kaiya, T.; Komatsu, N.; Ohsawa, N.; Fujimaki, M.; Tanuma, S.I.; Konno, K. Antimicrobial spectrum of lignin-related pine cone extracts of pinus parviflora Sieb. et Zucc. In Vivo 1990, 4, 7–12. [Google Scholar]
- Sláviková, E.; Košíková, B. Inhibitory effect of lignin by-products of pulping on yeast growth. Folia Microbiol. Off. J. Inst. Microbiol. Acad. Sci. Czech Repub. 1994, 39, 241–243. [Google Scholar] [CrossRef]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Cohen Hubal, E.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin—A biosorbent. J. Coll. Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, S.; Guo, X.; Huang, H. Adsorption of chromium (III) on lignin. Bioresour. Technol. 2008, 99, 7709–7715. [Google Scholar] [CrossRef]
- Harmita, H.; Karthikeyan, K.G.; Pan, X.J. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, S.; Shan, X.-Q. Adsorption of metal ions on lignin. J. Hazard. Mater. 2008, 28, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, C.; Rodríguez, J. EDTA: The chelating agent under environmental scrutiny. Quim. Nova 2003, 26, 901–905. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Li, C.; Ping, Q.; Shi, H.; Li, N.; Zhang, J.; Wang, C. A rapid and quantitative method for assessing the whiteness of whitened lignin based on an in-depth analysis of reported methods. Int. J. Biol. Macromol. 2020, 156, 1483–1490. [Google Scholar] [CrossRef]
- Vainio, U.; Maximova, N.; Hortling, B.; Laine, J.; Stenius, P.; Simola, L.K.; Gravitis, J.; Serimaa, R. Morphology of dry lignins and size and shape of dissolved kraft lignin particles by X-ray scattering. Langmuir 2004, 20, 9736–9744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, F.; Liu, X.; Fu, S. Micromorphology Influence on the Color Performance of Lignin and Its Application in Guiding the Preparation of Light-colored Lignin Sunscreen. ACS Sustain. Chem. Eng. 2018, 6, 12532–12540. [Google Scholar] [CrossRef]
- Lee, S.C.; Yoo, E.; Lee, S.H.; Won, K. Preparation and application of light-colored lignin nanoparticles for broad-spectrum sunscreens. Polymers 2020, 12, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, H.; Bai, Y.; Yu, B.; Liu, X.; Chen, F. A practicable process for lignin color reduction: Fractionation of lignin using methanol/water as a solvent. Green Chem. 2017, 19, 5152–5162. [Google Scholar]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Roh Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Sundqvist, B.; Karlsson, O.; Westermark, U. Determination of formic-acid and acetic acid concentrations formed during hydrothermal treatment of birch wood and its relation to colour, strength and hardness. Wood Sci. Technol. 2006, 40, 549–561. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Y.; Liu, Y.; Gao, J.; Chen, Y.; Fan, Y. Heat-induced discoloration of chromophore structures in Eucalyptus lignin. Materials 2018, 11, 1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Bai, Y.; Zhou, W.; Chen, F. Color reduction of sulfonated eucalyptus kraft lignin. Int. J. Biol. Macromol. 2017, 97, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Podschuna, J.; Saakea, B.; Lehnenb, R. Reactivity enhancement of organosolv lignin by phenolation for improved bio-based thermosets. Eur. Polym. J. 2011, 67, 1–11. [Google Scholar] [CrossRef]
- Matsushita, Y.; Yasuda, S. Preparation and evaluation of lignosulfonates as a dispersant for gypsum paste from acid hydrolysis lignin. Bioresour. Technol. 2005, 96, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Fu, S.; Chen, Y. High-value utilization of kraft lignin: Color reduction and evaluation as sunscreen ingredient. Int. J. Biol. Macromol. 2019, 133, 86–92. [Google Scholar] [CrossRef]
- Siddiqui, L.; Bag, J.; Seethab Mittal, D.; Leekha, A.; Mishra, H.; Mishra, M.; Verma, A.K.; Mishra, P.K.; Ekielski, A.; Iqbal, Z.; et al. Assessing the potential of lignin nanoparticles as drug carrier: Synthesis, cytotoxicity and genotoxicity studies. Int. J. Biol. Macromol. 2020, 152, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Xu, C. Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Tomani, P.E.R. The lignoboost process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]




| OCFs | UV Range | Photodegradation | Toxic Effects |
|---|---|---|---|
| AVOB | 357 | yes | Suppression of human trophoblast cells and apoptosis mediated by mitochondrial disruption [27], induction of obesogenic phenotypes [28], and hormone-like activity [29]. |
| HS | 295–315 | no | Skin penetration [30], disrupts estrogen [31]. |
| OMC | 280–355 | yes | Skin penetration, hormone like-activity; reproductive system, thyroid, and behavioral alterations in animal studies [17]. |
| OB | 270–350 | yes | Estrogenic activity, alteration of sperm production in the animal associated with endometriosis in women [32]. |
| Lignin Property | Petroleum Derived Compounds Substituted by Lignin | Ref. |
|---|---|---|
| Antioxidant | BHT, BHA | [66] |
| UV booster | acrylates/c10-30 alkyl acrylate cross-polymer | [67] |
| Antimicrobic agent | phenoxyethanol, hydroxybenzoates and triclosan | [68] |
| Chelating agent | EDTA, THPE | [69] |
| Emulsifier and stabilizer | acrylamides salts | [70] |
| Type | Grass Lignin | Softwood | Hardwood |
|---|---|---|---|
| H | 5–35% | <5% | 0–8% |
| G | 35–80% | >95% | 25–50% |
| S | 20–55% | 0% | 45–75% |
| Lignin | Tech. | Size (nm) | pH | Shape | Advantages | Application(s) | Limits | Ref. |
|---|---|---|---|---|---|---|---|---|
| SL | SEP | 50–250 | 7 | S | no aggregation | drug delivery and stabilizer | toxic chemicals | [109,110] |
| SL | AP | 50–250 | >7 | I | - | drug delivery, bioplastic | - | [111,112] |
| AL | AFR | 30–100 | <12 | S | high yield | bioplastic | aggregate | [94,113,114] |
| AL | AP | 30–100 | <12 | S | high stability | sunscreen | high ionic strength | [115] |
| AL | MR | 30–100 | <12 | S | dispersibility | antioxidant, antimicrobial | toxic chemicals | [116,117] |
| AL | SEP | 30–100 | <12 | S | - | antioxidant, antimicrobial | toxic chemicals | [115,118] |
| KL | AFR | 38–250 | 4–12 | I | stability | adhesives | - | [113,119] |
| KL | PCA | 38–250 | 4–12 | S | high solubility | commodities | high ionic strength | [120,121] |
| KL | FP | 38–250 | 4–12 | S | UV-shielding | materials | - | [110,122] |
| KL | MP | 38–250 | 4–12 | S | - | commodities | - | [110,122] |
| OL | AFR | 30–250 | 3.5–8 | S | high yield | adhesives | aggregate | [110,113,116] |
| OL | FP | 30–250 | 3.5–8 | S | stability | commodities | high ionic strength | [122] |
| OL | SEP | 30–250 | 3.5–8 | S | stability | materials | toxic chemicals | [112] |
| # | Name | Structure | ARP a | NRD b | # | Name | Structure | ARP | NRD |
|---|---|---|---|---|---|---|---|---|---|
| I | Guaiacol |  | 2.6 | 1.3 | VI | Guaiacyl propanol-1 |  | 3.0 | 1.5 |
| II | Syringol |  | 3.6 | 1.8 | VII | Coniferyl alcohol |  | 4.2 | 2.1 |
| III | Guacyl propanone-1 |  | 0.2 | <0.1 | VIII | Coniferyl aldehyde |  | 1.9 | 0.97 |
| IV | propiosyringone |  | 0.5 | 0.2 | IX | Isoeugenol |  | 2.2 | 1 |
| V | Propyl guaiacol |  | 3.5 | 1.75 | X | Eugenol |  | 4 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants 2021, 10, 274. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020274
Piccinino D, Capecchi E, Tomaino E, Gabellone S, Gigli V, Avitabile D, Saladino R. Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants. 2021; 10(2):274. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020274
Chicago/Turabian StylePiccinino, Davide, Eliana Capecchi, Elisabetta Tomaino, Sofia Gabellone, Valeria Gigli, Daniele Avitabile, and Raffaele Saladino. 2021. "Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications" Antioxidants 10, no. 2: 274. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020274