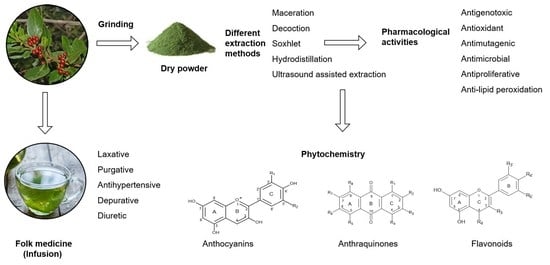
Rhamnus alaternus Plant: Extraction of Bioactive Fractions and Evaluation of Their Pharmacological and Phytochemical Properties
Abstract
:1. Introduction
2. Botanical Data
2.1. Geographical Distribution
2.2. Botanical Description
3. Extraction Processes Investigated in R. alaternus
3.1. Main Processes Applied
3.2. Maceration and Decoction
3.3. Soxhlet Extraction
3.4. Ultrasonic Assisted Extraction
3.5. Hydrodistillation
4. Phytochemistry
4.1. Generalities
4.2. Flavonoids
4.3. Anthraquinone Compounds
4.4. Anthocyanin Constituents
5. Biological Properties
5.1. Ethnopharmacology
5.2. Pharmacological Activities
5.2.1. Antihyperlipidemic Activity
5.2.2. Antioxidant Activity
| Parts of Plant | Analytical Method | Values | Country | Reference |
|---|---|---|---|---|
| Leaves | DPPH assay | 1.5–38 µg/mL equivalent vitamin C (fractions) | Tunisia | [50] |
| 12.60–90.81%; BHT is the positive control (fraction) | Algeria | [48] | ||
| 8.22 ± 0.01µg/mL; BHT is the positive control (extract) | Algeria | [42] | ||
| 07.76–38.87% (fractions) | Morocco | [47] | ||
| TEAC assay | 18.75–22.5 µg /mL equivalent of Trolox (fractions) | Tunisia | [103] | |
| 18.75 µg/mL 22.5 µg/mL equivalent of Trolox (fraction) | Tunisia | [24] | ||
| FRAP assay | 300–368 µg/mL equivalent of Trolox (fractions) | Tunisia | [31] | |
| Mixture of Leaves, Bark and Roots | DPPH assay | 2.35–58 µg/mL Vitamin E is the positive control (fraction) | Tunisia | [16] |
| Leaves Root | DPPH assay | 18.84 µg/mL 7.21 µg/mL, α-tocopherol is the positive control (extract) | Tunisia | [22] |
| Bark | DPPH assay ORAC assay FRAP assay TEAC assay | 0.39–0.61 mmol TE/g 3.96–6.55 mmol TE/g 1.24–1.72 mmol Fe2+/g 0.65–0.75 mmol TE/g (extract) | Algeria | [21] |
| DPPH assay β-carotene-linoleic acid assay Reducing power assay SRP Chelating activity | 78.7 ± 3.16 µg/mL 250 ± 6.84 µg/mL Ascorbic acid, quercetin and BHT are used as positive controls (extract) 0.91 ± 0.01 mg−1 1760 ± 60.7 µg/mL | Croatia | [8] | |
| Arial part | DPPH assay | 52.32–87.34% α-tocopherol is the positive control (extract) | Tunisia | [51] |
5.2.3. Antiproliferative Activity
5.2.4. Antimicrobial Activity
5.2.5. Toxicity
5.2.6. Antigenotoxic Activity
5.2.7. Antimutagenic Activity
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stocker, P.; Yousfi, M.; Djerridane, O.; Perrier, J.; Amziani, R. Effect of flavonoids from various Mediterranean plants on enzymatic activity of intestinal carboxylesterase. Biochimie 2004, 86, 919–925. [Google Scholar] [CrossRef]
- Lu, C.M.; Yang, J.J.; Wang, P.Y.; Lin, C.C. A new acylated flavonol glycoside and antioxidant effects of Hedyotis diffusa. Planta Med. 2000, 66, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.P.; Dumontet, V.; Van Tri, M.; Hill, B.; Thoison, O.; Se, T. Cytotoxicity of Rhamnosylanthraquinones and Rhamnosylanthrones from Rhamnus nepalensis. J. Nat. Prod. 2001, 64, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Končić, M.Z.; Kosalec, I.; Kremer, D. Anthraquinone profile, antioxidant and antimicrobial properties of bark extracts of Rhamnus catharticus and R. orbiculatus. Nat. Prod. Commun. 2011, 6, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Coskun, M.; Satake, T.; Hori, K.; Saiki, Y.; Tanker, M. Anthraquinone glycosides from Rhamnus Libanoticus. Phytochemistry 1990, 29, 2018–2020. [Google Scholar] [CrossRef]
- Chermat, S.; Gharzouli, R. Ethnobotanical Study of Medicinal Flora in the North East of Algeria—An Empirical Knowledge in Djebel Zdimm (Setif). J. Mater. Sci. Eng. A 2015, 5, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, M.S.; El-Toumy, S.A.A.; Merfort, I.; Nawwar, M.A.M. Polyphenolic metabolites of Rhamnus disperma. Phytochemistry 1999, 52, 943–946. [Google Scholar] [CrossRef]
- Kosalec, I.; Kremer, D.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Randić, M.; Zovko Končić, M. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem. 2013, 136, 335–341. [Google Scholar] [CrossRef]
- Locatelli, M.; Genovese, S.; Carlucci, G.; Kremer, D.; Randic, M.; Epifano, F. Development and application of high-performance liquid chromatography for the study of two new oxyprenylated anthraquinones produced by Rhamnus species. J. Chromatogr. A 2012, 1225, 113–120. [Google Scholar] [CrossRef]
- Lu, T.M.; Ko, H.H. A new anthraquinone glycoside from Rhamnus nakaharai and anti-tyrosinase effect of 6-methoxysorigenin. Nat. Prod. Res. 2016, 30, 2655–2661. [Google Scholar] [CrossRef]
- Gonçalves, R.S.; Silva, E.L.; Hioka, N.; Nakamura, C.V.; Bruschi, M.L.; Caetano, W. An optimized protocol for anthraquinones isolation from Rhamnus frangula L. Nat. Prod. Res. 2018, 32, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Genovese, S.; Epifano, F.; Curini, M.; Kremer, D.; Carlucci, G.; Locatelli, M. Screening for oxyprenylated anthraquinones in Mediterranean Rhamnus species. Biochem. Syst. Ecol. 2012, 43, 125–127. [Google Scholar] [CrossRef]
- Bas, J.M.; Oliveras, J.; Gómez, C. Myrmecochory and short-term seed fate in Rhamnus alaternus: Ant species and seed characteristics. Acta Oecol. 2009, 35, 380–384. [Google Scholar] [CrossRef]
- Tsahar, E.; Friedman, J.; Izhaki, I. Impact on Fruit Removal and Seed Predation of a Secondary Metabolite, Emodin, in Rhamnus alaternus Fruit Pulp. Oikos 2002, 99, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, J.M.; Rodriguez, R.; Rigueiro, C.; Hampe, A.; Jordano, P. Isolation and characterization of 12 microsatellite loci for Rhamnus alaternus (Rhamnaceae). Mol. Ecol. Resour. 2009, 9, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Ben Ammar, R.; Miyamoto, T.; Chekir-Ghedira, L.; Ghedira, K.; Lacaille-Dubois, M.-A. Isolation and identification of new anthraquinones from Rhamnus alaternus L. and evaluation of their free radical scavenging activity. Nat. Prod. Res. 2018, 33, 280–286. [Google Scholar] [CrossRef]
- Canale, A.; Benvenuti, S.; Raspi, A.; Benelli, G. Insect pollinators of the late winter flowering Rhamnus alaternus L., a candidate for honeybee-friendly scrubland spots in intensively managed agricultural areas. Plant Biosyst. 2014, 37–41. [Google Scholar] [CrossRef]
- Longo, L.; Vasapollo, G.; Rescio, L. Identification of anthocyanins in Rhamnus alaternus L. berries. J. Agric. Food Chem. 2005, 53, 1723–1727. [Google Scholar] [CrossRef]
- Miralles, J.; Martínez-Sánchez, J.J.; Franco, J.A.; Bañón, S. Rhamnus alaternus growth under four simulated shade environments: Morphological, anatomical and physiological responses. Sci. Hortic. 2011, 127, 562–570. [Google Scholar] [CrossRef]
- Ben Ammar, R.; Kilani, S.; Bouhlel, I.; Skandrani, I.; Naffeti, A.; Boubaker, J.; Ben Sghaier, M.; Bhouri, W.; Mahmoud, A.; Chekir-Ghedira, L.; et al. Antibacterial and cytotoxic activities of extracts from (Tunisian) Rhamnus alaternus (Rhamnaceae). Ann. Microbiol. 2007, 57, 453–460. [Google Scholar] [CrossRef]
- Boussahel, S.; Speciale, A.; Dahamna, S.; Amar, Y.; Bonaccorsi, I.; Cacciola, F.; Cimino, F.; Donato, P.; Ferlazzo, G.; Harzallah, D.; et al. Flavonoid profile, antioxidant and cytotoxic activity of different extracts from Algerian Rhamnus alaternus L. bark. Pharmacogn. Mag. 2015, 11, S102–S109. [Google Scholar] [CrossRef] [Green Version]
- Ammar, B.; Kilani, S.; Bouhlel, I.; Ezzi, L.; Skandrani, I.; Boubaker, J.; Ben Sghaier, M.; Naffeti, A.; Mahmoud, A.; Chekir-Ghedira, L.; et al. Antiproliferative, antioxidant, and antimutagenic activities of flavonoid-enriched extracts from (Tunisian) Rhamnus alaternus L.: Combination with the phytochemical composition. Drug Chem. Toxicol. 2008, 31, 61–80. [Google Scholar] [CrossRef]
- Tacherfiout, M.; Petrov, P.D.; Mattonai, M.; Ribechini, E.; Ribot, J.; Bonet, M.L.; Khettal, B. Antihyperlipidemic effect of a Rhamnus alaternus leaf extract in Triton-induced hyperlipidemic rats and human HepG2 cells. Biomed. Pharmacother. 2018, 101, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Bhouri, W.; Ben Sghaier, M.; Kilani, S.; Bouhlel, I.; Dijoux-Franca, M.G.; Ghedira, K.; Chekir Ghedira, L. Evaluation of antioxidant and antigenotoxic activity of two flavonoids from Rhamnus alaternus L. (Rhamnaceae): Kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside. Food Chem. Toxicol. 2011, 49, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef]
- Ferriol, M.; Llorens, L.; Gil, L.; Boira, H. Influence of phenological barriers and habitat differentiation on the population genetic structure of the balearic endemic Rhamnus ludovici-salvatoris Chodat and R. alaternus L. Plant Syst. Evol. 2009, 277, 105–116. [Google Scholar] [CrossRef]
- Penzig, O. Flore Coloriée de Poche du Littoral Méditerranéen de Gênes à Barcelone y Compris la Corse; Klincksieck, P., Ed.; Librairie Paris: Paris, France, 1902. [Google Scholar]
- Benarba, B. Medicinal plants used by traditional healers from South-West Algeria: An ethnobotanical study. J. Intercult. Ethnopharmacol. 2016, 5, 320–330. [Google Scholar] [CrossRef]
- Chancerel, L. Flore Forestière du Globe; Gauthier-Villars, H., Ed.; University of Michigan Library: Ann Arbor, MI, USA, 1920. [Google Scholar]
- Djidel, S.; Khennouf, S.; Baghiani, A.; Harzallah, D.; Arrar, L. Medicinal plants used traditionally in the algerian folk medicine for gastrointestinal disorders and hypertension: Total polyphenols, flavonoids and antioxidant activity. Acta Hortic. 2010, 854, 59–66. [Google Scholar] [CrossRef]
- Bhouri, W.; Boubaker, J.; Kilani, S. Flavonoids from Rhamnus alaternus L. ( Rhamnaceae ): Kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside protect against DNA damage in human lymphoblastoid cell and enhance antioxidant activity. S. Afr. J. Bot. 2012, 80, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Boudjelal, A.; Henchiri, C.; Sari, M.; Sarri, D.; Hendel, N.; Benkhaled, A.; Ruberto, G. Herbalists and wild medicinal plants in M’Sila (North Algeria): An ethnopharmacology survey. J. Ethnopharmacol. 2013, 148, 395–402. [Google Scholar] [CrossRef]
- Debeaux, M.O. Flore de la Kabylie du Djurdjura, ou, Catalogue Méthodique et Raisonné de Toutes les Plantes Vasculaires et Spontanées Observées Jusq’à ce jour Dans Cette Contrée; P. Klincksieck: Paris, France, 1894. [Google Scholar]
- Gubb, A.S. La flore Algérienne, Naturelle et Acquise; A. Jourdan: Alger, Algeria, 1913. [Google Scholar]
- Quezel, P.S.; Santa, S. Nouvelle Flore de l’Algérie et Des Régions Désertique Méridionales; Centre Nationale de la Recherche Scientifique: Paris, France, 1963. [Google Scholar]
- The Plant List, Version 1.1. 2013. Available online: http://www.theplantlist.org/ (accessed on 1 January 2021).
- Herrara, C.M. A Study of Avian Frugivores, Bird-Dispersed Plants, and Their Interaction in Mediterranean Scrublands. Ecol. Soc. Am. 1984, 54, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Bas, J.M.; Gómez López, C.; Pons, P. Morphological and structural characterization of evergreen buckthorn (Rhamnus alaternus L.) fruits in the northeastern Iberian Peninsula. Stud. Bot. 2002, 21, 89–103. [Google Scholar]
- El Aou-Ouad, H.; Florez-Sarasa, I.; Ribas-Carbo, M.; Flexas, J.; Medrano, H.; Gulias, J. Trade-offs between seedling growth, plant respiration and water-use efficiency in two Mediterranean shrubs Rhamnus alaternus and Rhamnus ludovici-salvatoris. Photosynthetica 2015, 53, 537–546. [Google Scholar] [CrossRef]
- Bas, J.M.; Pons, P.; Gómez, C.; Ecology, S.P.; Bas, J.M.; Pons, P. Exclusive Frugivory and Seed Dispersal of Rhamnus alaternus in the Bird Breeding Season breeding frugivory and seed dispersal season of Rhamnus alaternus in the bird. Plant Ecol. 2005, 183, 77–89. [Google Scholar] [CrossRef]
- Gulias, J.; Traveset, A.; Riera, N.; Mus, M. Critical Stages in the Recruitment Process of Rhamnus alaternus L. Ann. Bot. 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berka, B.; Hassani, A.; Allaf, K. Strategy of experimental design for intensification of solvent extraction of natural antioxidant flavonoids and phenols from buckthorn textured leaves. Cogent Chem. 2015, 1, 1–20. [Google Scholar] [CrossRef]
- Izhaki, I.D.O. Influence of nonprotein nitrogen on estimation of protein from total nitrogen in fleshy fruits. J. Chem. Ecol. 1993, 19, 2605–2615. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Zeouk, I.; Ouedrhiri, W.; Jiménez, I.A.; Lorenzo-morales, J.; Bazzocchi, I.L.; Bekhti, K.; Universitario, I.; Tropicales, D.E.; De Canarias, P.; De, U.; et al. Intra-combined antioxidant activity and chemical characterization of three fractions from Rhamnus alaternus extract: Mixture design. Ind. Crop. Prod. 2020, 144, 112054. [Google Scholar] [CrossRef]
- Moussi, K.; Nayak, B.; Perkins, L.B.; Dahmoune, F. HPLC-DAD profile of phenolic compounds and antioxidant activity of leaves extract of Rhamnus alaternus L. Ind. Crop. Prod. 2015, 74, 858–866. [Google Scholar] [CrossRef]
- Berroukche, A.; Kahloula, K.; Slimani, M.; Denai, I.; Ammour, K. Hepatoprotective effects of the decoction and macerated leaves of rhamnus alaternus L. On rats exposed to carbon tetrachloride. J. Pharmacogn. Phyther. 2015, 7, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Ben Ammar, R.; Bhouri, W.; Ben Sghaier, M.; Boubaker, J.; Skandrani, I.; Neffati, A.; Bouhlel, I.; Kilani, S.; Mariotte, A.M.; Chekir-Ghedira, L.; et al. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L. (Rhamnaceae): A structure-activity relationship study. Food Chem. 2009, 116, 258–264. [Google Scholar] [CrossRef]
- Ammar, B.; Kilani, S.; Abdelwahed, A.; Hayder, N.; Mahmoud, A.; Chibani, J.; Chekiri-Ghedira, L.; Ghedira, K. In vitro mutagenicity, antimutagenicity and free radical scavenging activities of Rhamnus alaternus L. (Rhamnaceae) Extracts. Pakistan J. Biol. Sci. 2005, 8, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Ben Ammar, R.; Neffati, A.; Skandrani, I.; Ben Sghaier, M.; Bhouri, W.; Ghedira, K.; Chekir-Ghedira, L. Anti-lipid peroxidation and induction of apoptosis in the erythroleukaemic cell line K562 by extracts from (Tunisian) Rhamnus alaternus L. (Rhamnaceae). Nat. Prod. Res. 2011, 25, 1047–1058. [Google Scholar] [CrossRef]
- Ben Ammar, R.; Ben Sghaier, M.; Boubaker, J.; Bhouri, W.; Naffeti, A.; Skandrani, I.; Bouhlel, I.; Kilani, S.; Ghedira, K.; Chekir-ghedira, L. Antioxidant activity and inhibition of aflatoxin B 1-, nifuroxazide-, and sodium azide-induced mutagenicity by extracts from Rhamnus L. Chem. Biol. Interact. 2008, 174, 1–10. [Google Scholar] [CrossRef]
- Berka, B.; Hassani, A.; Allaf, K.; Chemat, F. Analysis by Gas Chromatography-Mass Spectrometry of the Essential Oil of Rhamnus Alaternus L. (Rhamnaceae), an Aromatic and Medicinal Plant Growing in Algeria. J. Essent. Oil-Bearing Plants 2008, 11, 563–570. [Google Scholar] [CrossRef]
- Cuoco, G.; Mathe, C.; Vieillescazes, C. Liquid chromatographic analysis of flavonol compounds in green fruits of three Rhamnus species used in Stil de grain. Microchem. J. 2014, 115, 130–137. [Google Scholar] [CrossRef]
- Izhaki, I.; Tsahar, E.; Paluy, I.; Friedman, J. Within population variation and interrelationships between morphology, nutritional content, and secondary compounds of Rhamnus alaternus fruits. New Phytol. 2002, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Ben Ghezala, H.; Chaouali, N.; Gana, I.; Snouda, S.; Nouioui, A.; Belwaer, I.; Ouali, J.; Kaddour, M.; Masri, W.; Ben Salah, D.; et al. Toxic Effects of Rhamnus alaternus: A Rare Case Report. Case Rep. Emerg. Med. 2015, 2015, 182951. [Google Scholar] [CrossRef] [Green Version]
- Ammar, B.; Bouhlel, I.; Valenti, K.; Ben Sghaier, M.; Kilani, S.; Mariotte, A.-M.; Dijoux-Franca, M.-G.; Laporte, F.; Ghedira, K.; Chekir-Ghedira, L. Transcriptional response of genes involved in cell defense system in human cells stressed by H2O2 and pre-treated with (Tunisian) Rhamnus alaternus extracts: Combination with polyphenolic compounds and classic in vitro assays. Chem. Biol. Interact. 2007, 168, 171–183. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Azwanida, N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.D.L. De Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and emerging extraction processes of flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Belwal, T.; Huang, H.; Li, L.; Duan, Z.; Zhang, X.; Aalim, H. Optimization model for ultrasonic-assisted and scale-up extraction of anthocyanins from Pyrus communis ‘ Starkrimson ’ fruit peel. Food Chem. 2019, 297, 1–12. [Google Scholar] [CrossRef]
- Sukhdev Swami, H.; Suman Preet Singh, K.; Gennaro, L.; Dev Dutt, R. Extraction Technologies for Medicinal and Aromatic Plants; United Nations Industrial Development Organization and the International Centre for Science and High Technology: Trieste, Italy, 2008. [Google Scholar]
- Amiri, S.; Shakeri, A.; Sohrabi, M.; Khalajzadeh, S.; Ghasemi, E. Optimization of ultrasonic assisted extraction of fatty acids from Aesculus hippocastanum fruit by response surface methodology. Food Chem. 2019, 271, 762–766. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential oil composition. In Essential Oil Safety; © 2021 Robert Tisserand and Rodney Young; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; pp. 5–22. [Google Scholar] [CrossRef]
- Jian, C.Q. Spices and flavoring (flavouring) crops|Properties and Analysis. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 5491–5501. [Google Scholar]
- Grumezescu, A.M.; Holban, A.M. Ingredients Extraction by Physicochemical Methods in Food; Alexandru, M.G., Alina, M.H., Eds.; Elsevier: London, UK, 2017; ISBN 9780128115213. [Google Scholar]
- Zheljazkov, V.D.; Astatkie, T.; Schlegel, V. Hydrodistillation extraction time effect on essential oil yield, composition, and bioactivity of coriander oil. J. Oleo Sci. 2014, 63, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Chaar, C.I.; Kabbara, R.A.; Shamlian, S.N. A chromatographic study of the anthraquinones of rhamnus alaternus L. III. Extraction, isolation and chromatographic characterization of the anthraglycosides of the stem bark. Pharm. Biol. 1982, 20, 13–18. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Simpson, D.; Amos, S. Other Plant Metabolites. In Pharmacognosy; Elsevier Inc.: Cedarville, OH, USA, 2017; pp. 267–280. ISBN 9780128021040. [Google Scholar]
- Celli, G.B.; Tan, C.; Selig, M.J. Anthocyanidins and Anthocyanins. In Encyclopedia of Food Chemistry; Elsevier: New York, NY, USA, 2018; pp. 218–223. [Google Scholar]
- Goto, T.; Chemistry, O.; Agriculture, F. Structure, Stability and Color Variation of Natural Anthocyanins. In Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1987; pp. 113–158. ISBN 978-3-7091-8906-1. [Google Scholar]
- Zeouk, I.; Bekhti, K. A critical overview of the traditional, phytochemical and pharmacological aspects of Rhamnus alaternus: A Mediterranean shrub. Orient. Pharm. Exp. Med. 2019. [Google Scholar] [CrossRef]
- Inoue, T.; Hayashi, M.; Takayanagi, K.; Morooka, S. Lipid-lowering therapy with fluvastatin inhibits oxidative modification of low density lipoprotein and improves vascular endothelial function in hypercholesterolemic patients. Atherosclerosis 2002, 160, 369–376. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, C.; Yao, N.; Li, Y.; Wang, Q.; Fang, S.; Shang, X.; Zhao, M.; Che, C.; Ni, Y.; et al. Antihyperlipidemic effect of Cyclocarya paliurus (Batal.) Iljinskaja extract and inhibition of apolipoprotein B48 overproduction in hyperlipidemic mice. J. Ethnopharmacol. 2015, 166, 286–296. [Google Scholar] [CrossRef]
- Shattat, G.F. A review article on hyperlipidemia: Types, treatments and new drug targets. Biomed. Pharmacol. J. 2014, 7, 399–409. [Google Scholar] [CrossRef]
- Rodés, J.; Benhamou, J.-P.; Blei, A.T.; Reichen, J.; Rizzetto, M.; Dufour, J.-F.; Friedman, S.L.; Ginès, P.; Valla, D.-C.; Zoulim, F.; et al. Textbook of Hepatology: From Basic Science to Clinical Practice, 3rd ed.; Oxford University Press: Oxford, UK; Blackwell Publishing Ltd.: Oxford, UK, 2007; ISBN 9788578110796. [Google Scholar]
- Asztalos, B.F.; Schaefer, E.J. HDL in atherosclerosis: Actor or bystander? Atheroscler. Suppl. 2003, 4, 21–29. [Google Scholar] [CrossRef]
- National Cholesterol Education Program NCEP. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report." Circulation, 106, p. 3143; American Medical Association: Bethesda, MD, USA, 2002; Volume 106. [Google Scholar]
- Carlos, L.C.; Diego, A.S.; David, S.S.; Mahendra, R. Natural Antioxidants and Biocides from Wild Medicinal Plants; CABI: London, UK, 2013; ISBN 9781780642338. [Google Scholar]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Antioxidants and Antioxidant Enzymes in Higher Plants; Gewerbestrasse: Cham, Switzerland, 2018; ISBN 9783319750880. [Google Scholar]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A.M. Antioxidants: An Overview on the Natural and Synthetic Types. Eur. Chem. Bull. 2017, 6, 365. [Google Scholar] [CrossRef]
- Marc, F.; Davin, A.; Deglène-benbrahim, L.; Ferrand, C.; Baccaunaud, M.; Fritsch, P.; Marc, F.; Davin, A.; Deglène-benbrahim, L.; Ferrand, C.; et al. Studies of several analytical methods for antioxidant potential evaluation in food. M/S Méd. Sci. 2004, 20, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2010, 9, 217–233. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure-antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.Z.; Zhang, H.Y. Radical scavenging potential of phenolic compounds encountered in O. europaea products as indicated by calculation of bond dissociation enthalpy and ionization potential values. J. Agric. Food Chem. 2005, 53, 295–299. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Susanti, D.; Sirat, H.M.; Ahmad, F.; Ali, R.M.; Aimi, N.; Kitajima, M. Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L. Food Chem. 2007, 103, 710–716. [Google Scholar] [CrossRef]
- Spector, A. Review: Oxidative Stress and Disease. J. Ocul. Pharmacol. Ther. 2000, 16, 193–201. [Google Scholar] [CrossRef]
- Dziedzic, S.Z.; Hudson, B.J.F. Phenolic acids and related compounds as antioxidants for edible oils. Food Chem. 1984, 14, 45–51. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Lisbeth, A.; Noratto, G.; Hingorani, L.; Talcott, S.T.; Mertens-Talcott, S.U. Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J. Agric. Food Chem. 2008, 56, 8434–8441. [Google Scholar] [CrossRef]
- Chen, L.B.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Biochimie Antioxidant activity and phenolic pro fi le of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- Plaa, G.L.; Witshi, H. Chemicals, drugs, and lipid peroxidation. Annu. Rev. Pharmacol. Toxicol. 1976, 16, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Vasapollo, G.; Rescio, L.; Cuoco, G.; Mathe, C.; Vieillescazes, C.; Bhouri, W.; Ben Sghaier, M.; Kilani, S.; Bouhlel, I.; et al. Critical stages in the recruitment process of Rhamnus alaternus L. Acta Oecol. 2011, 87, 1167–1173. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics Annual 1996; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Ansary, J.; Gil, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ghellab, S.E.; Han, X. Lipid tubes formation induced by electroosmotic flow. Chem. Phys. Lett. 2018, 706, 515–519. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Stern, F.; England, N.; Mayer, J. Flavonoids: Dietary occurrence and biochemical activity. Nutr. Res. 1998, 18, 1995–2018. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.; Kim, J.; Hwang, J.K. Euchresta horsfieldii Benn. activates peroxisome proliferator-activated receptor α and regulates expression of genes involved in fatty acid metabolism in human HepG2 cells. J. Ethnopharmacol. 2011, 133, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Ghellab, S.E.; Li, Q.; Fuhs, T.; Bi, H.; Han, X. Electroformation of double vesicles using an amplitude modulated electric field. Colloids Surf. B Biointerfaces 2017, 160, 697–703. [Google Scholar] [CrossRef]
- Chen, G.; Wu, J.; Li, N.; Guo, M. Screening for anti-proliferative and anti-inflammatory components from Rhamnus davurica Pall. using bio-affinity ultrafiltration with multiple drug targets. Anal. Bioanal. Chem. 2018, 410, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Rui, H.L. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef]
- Ueda, J.Y.; Tezuka, Y.; Banskota, A.H.; Le Tran, Q.; Tran, Q.K.; Harimaya, Y.; Saiki, I.; Kadota, S. Antiproliferative activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2002, 25, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Dantas-Santos, N.; Almeida-Lima, J.; Vidal, A.A.J.; Gomes, D.L.; Oliveira, R.M.; Pedrosa, S.S.; Pereira, P.; Gama, F.M.; Rocha, H.A.O. Antiproliferative activity of fucan nanogel. Mar. Drugs 2012, 10, 2002–2022. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Michael, T.M.; John, M.M.; David, A.S.; David, P.C. Brok Bioloy of Microorganisms, 13th ed.; Pearson Education: San Francisco, CA, USA, 2012; ISBN 9780321649638. [Google Scholar]
- Heinrich, M.; Barnes, J.; Gibbons, S.; Williamson, E.M. Fundamentals of Pharmacognosy and Phytotherapy; Chirchil, L., Ed.; Elsevier: Edinburgh, UK, 2004. [Google Scholar]
- Gibbons, A. Exploring New Strategies to Fight Drug-Resistant Microbes. Science 1992, 257, 1036–1038. [Google Scholar] [CrossRef]
- Martini, N.D.; Katerere, D.R.; Eloff, J.N. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J. Ethnopharmacol. 2004, 93, 207–212. [Google Scholar] [CrossRef]
- Al-Qura’n, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Ohlan, D. In vitro studies on antifungal activity of tea (Camellia sinensis) and coffee (Coffea arabica) against wood-rotting fungi. Basic Microbiol. 1997, 37, 159–165. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Valgas, C.; De Souza, S.M.; Smânia, E.F.A.; Smânia, A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Michael, T.M.; Kelly, S.B.; Daniel, H.B.; Sattley, W.M.; David, A.S. Brock Biology of Microorganisms; Pearson: London, UK, 2019; ISBN 9781292235103. [Google Scholar]
- Djenane, D.; Yangüela, J.; Derriche, F.; Bouarab, L. Utilisation des composés de feuilles d ’ olivier comme agents antimicrobiens; application pour la conservation de la viande fraîche de dinde. Nat. Technol. 2012, 7, 53–61. [Google Scholar]
- Zeouk, I.; El Oouali Lalami, A.; Bekhti, K. In Vitro Antibacterial Activity of Medicinal Plants in the Central North of Morocco: A Possible Source of Alternative Drugs Against Methicillin-Resistant Staphylococcus Aureus. Asian J. Pharm. Clin. Res. 2019, 12, 285–292. [Google Scholar] [CrossRef]
- Li, L.; Mak, K.Y.; Shi, J.; Koon, H.K.; Leung, C.H.; Wong, C.M.; Leung, C.W.; Mak, C.S.K.; Chan, N.M.M.; Zhong, W.; et al. Comparative in vitro cytotoxicity study on uncoated magnetic nanoparticles: Effects on cell viability, cell morphology, and cellular uptake. J. Nanosci. Nanotechnol. 2012, 12, 9010–9017. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K. Quality Control and Evaluation of Herbal Drugs: Evaluating Natural Products and Traditional Medicine; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128133989. [Google Scholar]
- Elyebdri, N.; Boumediou, A.; Addoun, S. Ethnobotanical Study on the Usage of Toxic Plants in Traditional Medicine in the City Center of Tlemcen. Int. Sch. Sci. Res. Innov. 2017, 11, 642–646. [Google Scholar] [CrossRef]
- Dhawan, A.; Bajpayee, M. Genotoxicity Assessment Methods and Protocols; Springer: London, UK, 2013; ISBN 9781627035286. [Google Scholar]
- Jena, G.B.; Kaul, C.L.; Ramarao, P. Genotoxicity testing, a regulatory requirement for drug discovery and development: Impact of ICH guidelines. Indian J. Pharmacol. 2002, 34, 86–99. [Google Scholar]
- Swift, L.H.; Golsteyn, R.M. Genotoxic Anti-Cancer Agents and Their Relationship to DNA Damage, Mitosis, and Checkpoint Adaptation in Proliferating Cancer Cells. Int. J. Mol. Sci. 2014, 15, 3403–3431. [Google Scholar] [CrossRef] [Green Version]
- Quillardet, P.; Hofnung, M. The SOS Chromotest, a colorimetric bacterial assay for genotoxins: Procedures. Mutat. Res. Mutagen. Relat. Subj. 1985, 147, 65–78. [Google Scholar] [CrossRef]
- Quillardet, P.; Huisman, O.; D’Ari, R.; Hofnung, M. SOS chromotest, a direct assay of induction of an SOS function in Escherichia coli K-12 to measure genotoxicity. Proc. Natl. Acad. Sci. USA 1982, 79, 5971–5975. [Google Scholar] [CrossRef] [Green Version]
- Kocak, E. Investigation of potential genotoxic activity using the SOS Chromotest for real paracetamol wastewater and the wastewater treated by the Fenton process. J. Environ. Health Sci. Eng. 2015, 13, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mokdad-Bzeouich, I.; Kilani-Jaziri, S.; Mustapha, N.; Bedoui, A.; Ghedira, K.; Chekir-Ghedira, L. Evaluation of the antimutagenic, antigenotoxic, and antioxidant activities of Eriobotrya japonica leaves. Pharm. Biol. 2015, 53, 1786–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabchoub, F.; Messaâd, M.; Ben Mansour, H.; Chekir-Ghedira, L.; Salem, M. Synthesis and antigenotoxic activity of some naphtho[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives. Eur. J. Med. Chem. 2007, 42, 715–718. [Google Scholar] [CrossRef]
- Berhow, M.A.; Wagner, E.D.; Vaughn, S.F.; Plewa, M.J. Characterization and antimutagenic activity of soybean saponins. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2000, 448, 11–22. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Waters, M.D.; Stack, H.F.; Jackson, M.A.; Brockman, H.E.; De Flora, S. Activity profiles of antimutagens: In vitro and in vivo data. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1996, 350, 109–129. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Zani, F.; Cuzzoni, M.T.; Daglia, M.; Benvenuti, S.; Vampa, G.; Mazza, P. Inhibition of Mutagenicity in Salmonella typhimurium by Glycyrrhiza glabra: Extract, glycyrrhizinic acid, 18a- and 18b-glycyrrhetinic acids. Planta Med. 1993, 59, 502–507. [Google Scholar] [CrossRef]

| Part of Plant | Extraction Method | Solvent(s) Used | Bioactive Compounds (or Groups) | Pharmacological Activities | Reference | |
|---|---|---|---|---|---|---|
| Maceration | Methanol | Kaempferol hexoside, rhamnocitrin, kaempferol 3-O acetyl-rhamnoside, quercetin, pilosin hexoside, pilosin, apigenin glucoside, rhamnocitrin hexoside, rhamnetin hexoside, kaempferol | Antihyperlipidemic acitivity | [23] | ||
| Ethanol, distillated water, methanol, ethyl acetate | Emodin, kaempferol | Antioxidant activity | [47] | |||
| Methanol | Rutin, antraquinones, quercetin-3-rhamnoside, kaempferol, gallic acid, p-coumaric acid, ferulic acid, luteolin | Antioxidant activity | [48] | |||
| Maceration/ Decoction | Distilled water | NA | Hepatoprotective effects | [49] | ||
| Soxhlet extraction/ Maceration | Methanol, petroleum ether, chloroform, ethyl acetate water/acetone | Rhamnetin-3-O-isorhamninoside, kaempferol 3-O-isorhamninoside, rhamnocitrin- 3-O-isorhamninoside | Antioxidant activity | [50] | ||
| Leaves | Methanol, chloroform, petroleum ether, ethyl acetate water, acetone | Kaempferol 3-O-β-isorhamninoside, rhamnocitrin 3-O-β-isorhamninoside | Antioxidant activity Antigenotoxic activity | [24] | ||
| Petroleum ether, chloroform, ethyl acetate, methanol, butanol water, acetone | Coumarins, flavonoids, anthraquinones, tannins | Antimicrobial activity | [20] | |||
| Petroleum ether, chloroform, ethyl acetate, methanol, water, acetone | Flavonoids, anthraquinones, tannins, sterols, coumarins | Antigenotoxic activity Antimutagenic activity | [51] | |||
| Ethyl acetate, water, acetone, chloroform | Flavonoids | NA | [52] | |||
| Soxhlet extraction | Chloroform, water, petroleum ether, ethyl acetate, dimethyl sulfoxide, butanol | Flavonoids, phenols | Antigenotoxic activity Antimutagenic activity | [53] | ||
| Hydrodistillation extraction | Water | Oxygenated monoterpenes hydrocarbons, oxygenated diterpenes hydrocarbons, oxygenated Sesquiterpenes hydrocarbons, sesquiterpenes hydrocarbons, monoterpenes hydrocarbons, aliphatic hydrocarbons, fatty acids | NA | [54] | ||
| Berries | Maceration | Methanol | Malvidin, delphinidin 3-rutinoside, cyandin, petunidin 3-glucoside, petunidin, delphinidin 3-glucoside, pelagonidin, malvidin 3-rutinoside, peonidin 3-rutinoside, peonidin, cyanidin 3-rutinoside, delphindin peonidin 3-glucoside, malvidin 3-glucoside, cyanidin 3-glucoside, petunidin 3-rutinoside, pelargonidin 3-rutinoside, | NA | [18] | |
| Methanol, water | Quercetin, rhamnazin-3-O rhamninoside, rhamnazin, quercetin-4′-O-rhamninoside, rhamnetin, kaempferol-4′-O-rhamninoside, isorhamnetin, rhamnocitrin, kaempferol, rhamnocitrin-3-O-rhamninoside, quercetin-3-O-rhamninoside, rhamnetin-3-O-rhamninoside, rhamnocitrin-4′-O-rhamninoside, kaempferol-3-O-rhamninoside | NA | [55] | |||
| Soxhlet extraction | Petroleum ether, methanol | Emodin | NA | [56] | ||
| Bark | Maceration/Decoction | Methanol, water | Quercetin, kaempferol, rhamnocitrin, rhamnetin, | Antioxidant activity | [21] | |
| Ultrasonic extraction | Methanol, ethyl acetate | Flavonoids, aloe-emodin, rhein, emodin, chrysophanol, physcion | Antioxidant activity Antimicrobial activity | [8] | ||
| Roots | Decoction | Chloroform, dichloromethane, ethyl acetate | Rhein, physcion, aloe-emodin | NA | [57] | |
| Leaves root bark | Maceration | Methanol, butanol | Flavonoids, coumarins, anthraquinones, sterols | Antiproliferative activity Antimutagenic activity | [22] | |
| NA | NA | Emodin- 6-O-α-L-rhamnoside, β-sitosterol, physcion-8-O-rutinoside, kaempferol-7-methylether. 1, 6 dihydroxy-3 methyl 6 [2′-Me (heptoxy)] anthraquinone. β-sitosterol-3-O-glycoside. 1,4,6,8 tetrahydroxy-3 methyl anthraquinone 1-O-β-D-glucopyranosyl-4,6-di-O-α-L rhamnopyranoside. 1,2,6,8 tetrahydroxy-3 methyl anthraquinone 8-O-β-D-glucopyranoside | Antioxidant activity | [16] | ||
| Aerial part | Soxhlet extraction/Maceration | Methanol, ethyl acetate, chloroform water, acetone | Flavonoids, tannins | Antigenotoxic activity | [58] | |
| Compound Class | Compound | Compound Number * | Reference |
|---|---|---|---|
| Flavonoids | Quercetin-3-O-rhamninoside | 1 | [48,55] |
| Kaempferol-3-O-rhamninoside | 2 | [55] | |
| Quercetin-4′-O-rhamninoside | 3 | [55] | |
| Kaempferol-4′-O-rhamninoside | 4 | [55] | |
| Rhamnetin-3-O-rhamninoside | 5 | [55] | |
| Rhamnocitrin-3-O-rhamninoside | 6 | [55] | |
| Rhamnocitrin-4′-O-rhamninoside | 7 | [55] | |
| Kaempferol | 8 | [21,23,47,48,55] | |
| Quercetin | 9 | [21,23,55] | |
| Isorhamnetin | 10 | [21,55] | |
| Rhamnetin | 11 | [21,55] | |
| Rhamnazin | 12 | [55] | |
| Kaempferol-3-O-isorhamninoside | 13 | [23,24,50] | |
| Rhamnocitrin-3-O-isorhamninoside | 14 | [24,50] | |
| Rhamnetin-3-O-isorhamninoside | 15 | [50] | |
| Anthraquinones | Emodin | 16 | [8,47,56] |
| Rhein | 17 | [8,57] | |
| Chrysophanol | 18 | [8] | |
| Physcion | 19 | [8,57] | |
| 1,4,6,8 tetrahydroxy-3 methyl anthraquinone | 20 | [16] | |
| 1-O-β-D-glucopyranosyl-4,6-di-O-α-L-rhamnopyranoside | |||
| 1,2,6,8 tetrahydroxy-3 methyl anthraquinone 8-O-β-D-glucopyranoside | 21 | [16] | |
| 1, 6 dihydroxy-3 methyl 6 [2′-Me (heptoxy)] anthraquinone | |||
| Physcion-3-O-β-rutinoside | 22 | [16] | |
| Emodin-6O-α-L-rhamnopyranoside | 23 | [16] | |
| β-sitosterol | 24 | [16] | |
| β-sitosterol-3-O-β-D-glycopyranoside | 25 | [16] | |
| 26 | [16] | ||
| Anthocyanins | Cyanidin 3-rutinoside | 27 | [18] |
| Petunidin 3-rutinoside | 28 | [18] | |
| Delphinidin 3-rutinoside | 29 | [18] | |
| Pelargonidin 3-rutinoside | 30 | [18] | |
| Peonidin 3-rutinoside | 31 | [18] | |
| Malvidin 3-rutinoside | 32 | [18] | |
| Delphinidin 3-glucoside | 33 | [18] | |
| Cyanidin 3-glucoside | 34 | [18] | |
| Petunidin 3-glucoside | 35 | [18] | |
| Pelargonidin 3-glucoside | 36 | [18] | |
| Peonidin 3-glucoside | 37 | [18] | |
| Malvidin 3-glucoside | 38 | [18] | |
| Delphindin | 39 | [18] | |
| Cyandin | 40 | [18] | |
| Petunidin | 41 | [18] | |
| Pelagonidin | 42 | [18] | |
| Peonidin | 43 | [18] | |
| Malvidin | 44 | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekkaa, A.; Benaissa, A.; Mutelet, F.; Canabady-Rochelle, L. Rhamnus alaternus Plant: Extraction of Bioactive Fractions and Evaluation of Their Pharmacological and Phytochemical Properties. Antioxidants 2021, 10, 300. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020300
Nekkaa A, Benaissa A, Mutelet F, Canabady-Rochelle L. Rhamnus alaternus Plant: Extraction of Bioactive Fractions and Evaluation of Their Pharmacological and Phytochemical Properties. Antioxidants. 2021; 10(2):300. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020300
Chicago/Turabian StyleNekkaa, Amine, Akila Benaissa, Fabrice Mutelet, and Laetitia Canabady-Rochelle. 2021. "Rhamnus alaternus Plant: Extraction of Bioactive Fractions and Evaluation of Their Pharmacological and Phytochemical Properties" Antioxidants 10, no. 2: 300. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10020300