1. Introduction
Osteoarthritis (OA) is a degenerative joint disease, a leading cause of chronic disability, and an important national health issue [
1,
2]. In roughly half of afflicted individuals, OA restricts normal daily activities and greatly reduces quality of life. OA can affect the entire joint structure, including articular cartilage, subchondral bone, synovium, tendons, muscle, and meniscus fibrocartilage tissue in the knee joints [
3,
4]. The disease has been attributed to an imbalance between the synthesis and degradation of the extracellular matrix (ECM) [
5,
6]. Furthermore, increased inflammatory mediators in joints during OA progression contributes to the destruction of the cartilage matrix [
4]. Nonetheless, recent cartilage repair treatments have the potential to not only relieve pain and improve quality of life but also to delay or eliminate the need for joint replacement [
7]. Most approaches to OA management focus on pain relief using systemic or local drugs, physical therapies, or surgery [
8]. However, current therapeutic options have a number of major drawbacks, such as applicability to limit size defect, long rehabilitation times, and a lack of readily available graft material [
9,
10].
Inflammatory cytokines and catabolic factors hinder the functions of chondrocytes and promote the development of OA [
3]. One inflammatory cytokine, interleukin-1 beta (IL-1β) has been identified in osteoarthritic synovial fluid and can trigger a succession of cartilage related catabolic effects [
11]. This process directly activates nuclear factor kappa-B (NF-κB) and mitogen-activated protein (MAP) kinase, which up-regulates the expression of cartilage matrix-degrading enzymes, such as cyclooxygenase 2 (COX2), inducible nitric oxide synthase (iNOS), and matrix metalloproteinases (MMPs) [
12]. These cytokines are elevated in joint disorders and are closely related to pathological conditions that lead to the production of nitric oxide (NO) and prostaglandin E
2 (PGE
2). Previous research identified NO and PGE
2 (both of which are highly expressed in the synovial fluid of OA patients) as therapeutic targets in the treatment of OA [
13,
14]. However, there are many adverse effects associated with non-steroidal anti-inflammatory drugs (NSAIDs) [
15,
16]. Thus, inhibiting IL-1β-stimulated inflammatory mediators via natural sources is the preferred approach to OA treatment.
In recent years, natural plant extracts have been demonstrated to have anti-inflammatory and antioxidant effects. Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) is a chalcone isolated primarily from Zingiberaceae which has been reported to exert anti-inflammatory and antioxidant properties [
17]. Cardamonin interacts with various cellular signaling targets, such as nuclear erythroid 2-related factor 2 (Nrf2), extracellular signal-regulated kinase (ERK), and mammalian target of rapamycin (mTOR), during the progression of tumors [
18]. These interactions provide evidence that the mechanism behind the anti-tumor activities of cardamonin involves negatively modulating the signal transducer and activator of transcription (STAT) family [
19]. Cardamonin has also recently been reported to attenuate the expression of COX2 and iNOS by reducing p65/nuclear factor-κB (NF-κB) nuclear translocation and inhibiting phosphorylation of nuclear factor kappa B (IκB) [
20]. Our objectives in the current study were to investigate the protective effects of cardamonin on human chondrocytes in OA models and to identify the mechanism which underlies these protective effects.
2. Materials and Methods
2.1. Primary Culture
Human articular cartilage tissues were obtained from OA patients who underwent total knee replacement. Residual osteoarthritic cartilage was first removed from the joint surfaces, and articular cartilage was then cut into small fragments and incubated with antimicrobial solution (500 IU/mL penicillin/streptomycin) (Gibco, Carlsbad, CA, USA) for 3 h. Subsequently, articular cartilage was washed with phosphate buffered saline (PBS) (Gibco, Carlsbad, CA, USA) and digested. Articular chondrocyte extraction was then performed via sequential enzymatic digestion at 37 °C under 5% CO2 with 0.25% trypsin (Gibco, Carlsbad, CA, USA) for 30 min followed by 3 mg/mL blend collagenase type H (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 12 h. The samples were checked for digestion by examination under a BX43 light microscope (Olympus Corp, Tokyo, Japan), and the cell suspension was collected and filtered through nylon mesh using a sterile pastette. After centrifugation at 1000 rpm for 10 min, the supernatant was discarded, and the pellet was resuspended in 10 mL Dulbecco’s Modified Eagle’s medium (DMEM)/Nutrient Mixture F-12 HAM medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), 100 international units (IU)/mL penicillin, and 100 µg/mL streptomycin. Finally, the pellet was cultured under a humidified 5% CO2 atmosphere at 37 °C. Cells between passages 2 and 3 were used in subsequent experiments.
2.2. Cell Viability
Chondrocyte viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche, Indianapolis, IN, USA). For this, primary human OA chondrocytes were seeded in 200 µL complete medium in a 96-well plate at a density of 10,000 cells per well. At confluence, chondrocytes were serum starved overnight and then treated with various concentrations of cardamonin (1, 5, 10, 20, 40 µg/mL) (Catalog number: C8249; Sigma-Aldrich, St. Louis, MO, USA) in serum free medium for 24 or 48 h. Chondrocytes were subsequently treated with 20 µl of MTT at 0.5 mg/mL (Sigma-Aldrich, St. Louis, MO, USA) for 3 h and formazan crystal solubilized using dimethyl sulfoxide (DMSO) at 150 µL (Sigma-Aldrich, St. Louis, MO, USA). Absorbance was recorded at 570 nm using a Synergy HT plate reader (Bio-Tek Instruments Inc., Winooski, VT, USA).
2.3. Reactive Oxygen Species (ROS) Measurement
ROS was measured via 2′,7′-dichlorofluorescein-diacetate (H2DCF-DA) (Sigma-Aldrich, St. Louis, MO, USA) staining in accordance with the manufacturer’s instructions and then observed using a microscope. For the measurement of IL-1β induced ROS, chondrocytes were pretreated with SBE fractions (50 µg/mL) for 2 h, labeled with H2DCF-DA (20 µM) for 0.5 h, and then stimulated with IL-1β for 5 min. Detailed protocol for ROS detection has been previously described by Khan et al. [
21].
2.4. Griess Reaction
NO concentrations in synovial fluid can be derived from their stable end product, nitrite, measured using the Griess reaction. Briefly, we incubated an aliquot of joint fluid or cultured medium with 50 µL of 1% sulphanilamide (Sigma-Aldrich, St. Louis, MO, USA) in 5% phosphoric acid (Sigma-Aldrich, St. Louis, MO, USA) and 50 µL of 0.1% N-1-naphthylethylenediamine dihydrochloride (Sigma-Aldrich, St. Louis, MO, USA). After 20 min incubation at room temperature, absorbance was using a microplate reader to measure at a wavelength of 550 nm (BioTek Instruments, Winooski, VT, USA).
2.5. Extraction of Protein and Western Blotting Analysis
Both of the cells and tissues were immediately washed using PBS and lysed in situ for 15 min with radioimmunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher Pierce, Waltham, MA, USA) containing 100 µM Na
3VO
4, and 100× protease inhibitor cocktail (Thermo Fisher Pierce, Waltham, MA, USA). Following centrifugation for 15 min at 13,000 rpm, whole cell lysates were collected, and the protein concentration was determined using the Lowry method. Equal amounts of protein were then loaded onto 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to polyvinylidene fluoride (PVDF) membranes (Merck KGaA, Darmstadt, Germany). PVDF membranes were incubated with bovine serum albumin (BSA) at 2%. (Sigma-Aldrich, St. Louis, MO, USA) in TBST (12.5 mM Tris/HCl, pH 7.6, 137 mM NaCl, 0.1% Tween 20) (Sigma-Aldrich, St. Louis, MO, USA) at 4 °C overnight. After washing with TBST three times, blots were incubated with primary antibodies diluted in TBST. After washing with TBST three more times, the blots were incubated with horseradish peroxidase (HRP) labeled secondary antibodies at room temperature for 1 h. Membranes were then rewashed thoroughly, and the binding results were detected using the enhanced chemiluminescence plus Western blotting detection system (Thermo Fisher Pierce, Waltham, MA, USA) in accordance with the manufacturer’s instructions. Finally, membranes were scanned and subjected to densitometry analysis (VisionWorks LS, UVP, CA, USA) in accordance with the manufacturer’s protocols. Specific antibodies that we used are listed in
Supplementary Table S1.
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
To detect cytokine expression levels in tissue fluid and cultured medium in accordance with the manufacturer’s protocols we used an ELISA kit (R&D Systems, Minneapolis, MN, USA).
2.7. Gelatin Zymography
The extract was mixed with sample buffer solution containing SDS, glycerol, and bromophenol blue (Sigma-Aldrich, St. Louis, MO, USA, respectively). Equal quantities of each sample were separated on SDS-polyacrylamide gel (8%) containing 1 mg/mL gelatin (Sigma-Aldrich, St. Louis, MO, USA). After performing SDS-polyacrylamide gel electrophoresis, the gels were washed twice using 2.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 30 min to remove SDS and then twice more with distilled water. The gels were subsequently equilibrated with incubation buffer (100 mM Tris/HCl, 30 mM CaCl2, 0.01% NaN3) (Sigma-Aldrich, St. Louis, MO, USA) and then incubated with incubation buffer for 20 h at 37 °C prior to staining with Coomassie Blue solution for 40 min. Destaining was performed in methanol/acetic acid/distilled water.
2.8. Safranin O-Fast Green Staining
Slides were preheated for 30 min at 75 °C, then dewaxed and hydrated in an ethanol gradient of 70%, 80%, 95%, or 100% (Sigma-Aldrich, St. Louis, MO, USA). Following this, slides were stained with Weigert’s iron hematoxylin working solution (Sigma-Aldrich, St. Louis, MO, USA) for 5 min at room temperature and washed gently under running tap water for 5 min before being stained with 0.02% Fast Green solution (Sigma-Aldrich, St. Louis, MO, USA) for 5 min. Slides were then rinsed in 1% acetic acid (Sigma-Aldrich, St. Louis, MO, USA) for 10 s then transferred to 1% safranin O solution (Sigma-Aldrich, St. Louis, MO, USA) for 3 min. Finally, slides were carefully rinsed twice in fresh 95% ethanol and air dried.
2.9. Electrophoretic Mobility Shift Assay (EMSA)
The DNA binding activity of NF-κB, AP-1, and STAT-3, EMSA was evaluated using a LightShift Chemiluminescence RNA EMSA Kit (Thermo Fisher Scientific, Waltham, MA, USA). For this, biotin end-label duplex DNA was incubated with nuclear extract and electrophoresed on native gel. The reaction mixtures were separated using 5% native polyacrylamide gel at 4 °C for 1 h before being transferred to a nylon membrane (GE Healthcare, Buckinghamshire, UK). Biotin end-labeled DNA was tested with a streptavidin–HRP conjugate and enhanced chemiluminescence (ECL) reagents. Finally, the membranes were exposed to UV light for 10 min and analyzed using the UVP AutoChemi Image System (UVP Inc., Upland, CA, USA).
2.10. SiRNA Transfection
We obtained siNrf2 and control siRNA from Applied Biosystems (Thermo Fisher Pierce, Waltham, MA, USA). Primary chondrocyte cells were seeded in 6-well plates and transfected with siNrf2 or control siRNA using lipofectamine 2000 reagent (Invitrogen, Waltham, MA, USA) in accordance with the manufacturer’s instructions. The cells were then cultured for 24 h prior to use in the experiments.
2.11. Measurement of ROS and Immunofluorescence Assay
Quantification of the inhibitory effect of cardamonin upon cellular oxidative stress was incubated with H2DCF-DA for 30 min which is a cell-permeable probe used to detect intracellular ROS. Following incubation, cells were gently washed twice with PBS to remove unbound H2DCF-DA. Cells were then cultured with IL-1β in chamber slides at a density of 1 × 104 cells for 5 min. To measure the intracellular expression of ROS, we observed emissions under excitation at a wavelength of 485 nm using an BX61 immunofluorescence microscope (Olympus Corp, Tokyo, Japan).
2.12. Statistical Analysis
All statistical analysis was conducted using Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) and Image J software (NIH, Bethesda, MD, USA). All data were obtained from at least three independent experiments, and values were expressed as mean ± standard deviation (SD). Statistical evaluation of quantification data pertaining to mRNA and protein was performed using Student’s t-tests. The results were considered significant at a p-value less than 0.05.
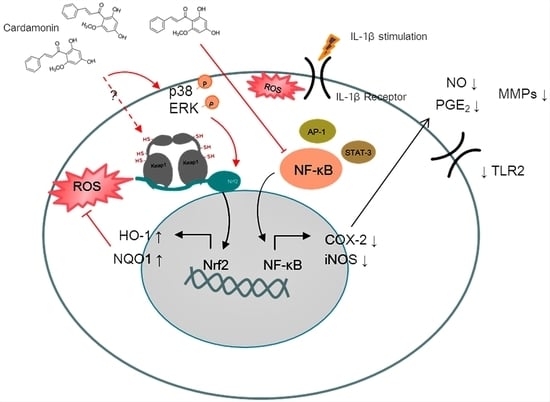
4. Discussion
OA is a complex joint pathology that leads to chronic disability. The precise mechanism of OA pathogenesis has not been elucidated, and no effective treatment has been developed to block the progression of the disease. Increasing evidence suggests that cardamonin possesses anti-inflammatory and antioxidant properties. This study reports two major findings. Firstly, cardamonin inhibited the overexpression of iNOS (
Figure 1B,C), COX2 (
Figure 1B,D), and MMPs (
Figure 2) in chondrocytes stimulated by IL-1β. Secondly, cardamonin reduced the generation of ROS in chondrocytes (
Figure 3C,D) by activating the Nrf2 pathway (
Figure 4,
Figure 5 and
Figure 6) via p38 (
Figure 6A,C) and ERK (
Figure 6A,D). Previous studies have reported that the mediators secreted in the early stages of OA are proinflammatory cytokines, including IL-1β and tumor necrosis factor-alpha (TNF-α). This evidence shows that IL-1β plays a central role in the pathogenesis of OA; therefore, it is commonly used to induce OA in vitro in chondrocytes. IL-1β stimulation induces the expression of COX2, which in turn increases the synthesis of PGE2. PGE2 has been implicated in bone resorption and joint pain in cases of OA [
22]. PGE2 and NO are both capable of upregulating the production of MMPs and other inflammatory cytokines, and inhibiting the IL-1β-stimulated production of inflammatory mediators has proven useful in the treatment of OA. In this study, pretreatment with cardamonin significantly decreased IL-1β-stimulated PGE2 (
Figure 1E) and NO (
Figure 1F) production by attenuating the expression of iNOS (
Figure 1B,C) and COX2 (
Figure 1B,D). These observations indicate that the expression of iNOS and COX2 could be regulated by the anti-inflammatory effects of cardamonin in articular chondrocytes.
OA is characterized by the destruction of articular cartilage due to an imbalance between the biosynthesis and degradation of the extra cellular matrix (ECM). Previous studies have demonstrated that MMPs play a crucial role in the degradation of articular cartilage. MMPs are proteolytic enzymes commonly expressed in joint disorders. They have been shown to degrade the ECM and are therefore viewed as a promising pharmacological target for the treatment of OA. The fact that MMP-1, MMP-3, and MMP-13 are primarily found in cartilage indicates their probable participation in OA progression. We therefore sought to determine whether cardamonin could be used to inhibit the expression and/or activity of MMPs and inflammatory mediators induced in chondrocytes by IL-1β. MMP-2 and MMP-9 activity was investigated using gelatin zymography in order to examine the effects of cardamonin treatment following IL-1β stimulation (
Figure 2A). In previous studies, IL-1β was shown to induce the expression of MMP-1, MMP-3, MMP-13, and ADAMTS-4 in human tendon cells [
23]. In the current study, we detected the IL-1β induced expression of MMP-1, MMP-2, MMP-3, MMP-9, and MMP-13 in articular chondrocytes (
Figure 2). For MMP-9 activities detection, IL-1β showed a significant increase after 24 h treatment. However, when cells were pretreated with cardamonin prior to IL-1β stimulation, the production of MMP-1 (
Figure 2D), MMP-3 (
Figure 2E), and MMP-13 (
Figure 2F) was significantly decreased compared with cells that were only stimulated with IL-1β and did not receive cardamonin pretreatment. Cardamonin was also shown to suppress the activities of MMP-2 and MMP-9 (
Figure 2A,B). This suggests that cardamonin (1) exerts protective effects on chondrocytes and (2) has a potential role in treating cartilage damage in cases of OA.
ROS are small reactive molecules derived from molecular oxygen. They have been linked to oxidative stress and inflammatory response in OA [
24]. ROS degrades a variety of GAGs, such as chondroitin sulfate (CS), hyaluronic acid (HA), and dermatan sulfate [
25]. Potential sources of ROS identified in chondrocytes, such as NO synthase, can modulate chondrocyte behavior and extracellular matrix homeostasis in OA patients. In the current study, we investigated the effects of cardamonin on the activation of iNOS and ROS in IL-1β-stimulated chondrocytes with the aim of elucidating the mechanism which underlies the antioxidation effects of cardamonin. In previous studies, IL-1β-stimulated chondrocytes were shown to induce high iNOS and ROS levels. In the current study, pretreatment with cardamonin attenuated IL-1β-stimulated iNOS activation (
Figure 2B,C) and ROS expression (
Figure 3C,D) in chondrocytes.
Nrf2 is a member of the cap ‘n’ collar (CNC) transcription factor subfamily [
26]. Previous studies which employed Nrf2-knockout mice reported increased mRNA and protein levels for COX2, iNOS, interleukin-6 (IL-6), and TNF-α. Nonetheless, the activation of Nrf2 could lead to nuclear translocation, which should decrease the level of COX2 and iNOS expression. Research has shown that Nrf2-dependent antioxidant genes HO-1 and NQO-1 can block TNF-α and IL-6 inflammatory mediators. Previous studies have also reported that HO-1 expression can be enhanced by various oxidative-inducing lipopolysaccharides (LPS) [
27]. We found that cardamonin inhibited the expression of inflammatory proteins (COX2 and iNOS) and inflammatory cytokines (PGE2) while increasing the expression of antioxidant proteins HO-1 and NQO1. We also found that cardamonin treatment dramatically inhibited the increase in HO-1 levels induced by IL-1β (
Figure 4C). For IL-1β stimulation, overproduction or overexpression of HO-1 (
Figure 5A,C) was reduced with the IL-1β-stimulated ROS in the articular chondrocyte culture medium (
Figure 6A,B). Taken together, these results indicate that the anti-inflammatory effects of cardamonin can be attributed to activation of the Nrf2 pathway.
High iNOS, COX2, and MMP expression levels in arthritic joints is due to the activation of a tightly regulated and synchronized signaling cascade which is activated by IL-1β and involves the mitogen-activated protein kinases (MAPKs) signaling pathway [
28,
29]. Using cardamonin to suppress NO generation via iNOS phosphorylation delayed the activation of p38 and ERK1/2 kinases. Recent studies have shown that NO activates ERK signaling through the down-regulation of MAP kinase phosphatase. MAPKs are activated through the phosphorylation of specific tyrosine and threonine residues by upstream kinases in response to inflammatory signals. In the current study, we demonstrated that IL-1β enhances the translocation of NF-ΚB and the activation of the p38 and ERK signaling pathways (
Figure 7C,D). Interestingly, pretreating chondrocyte cells with cardamonin suppressed the IL-1β-induced release of MMPs, iNOS, and PGE2, as indicated by the attenuation of their corresponding gene expressions. This was at least partly achieved through the blocking of NF-κB and p38 activation. These findings suggest that cardamonin could be a novel Nrf2 activator and a useful nutritional supplement in OA therapy.