The Overactivation of NADPH Oxidase during Clonorchis sinensis Infection and the Exposure to N-Nitroso Compounds Promote Periductal Fibrosis
Abstract
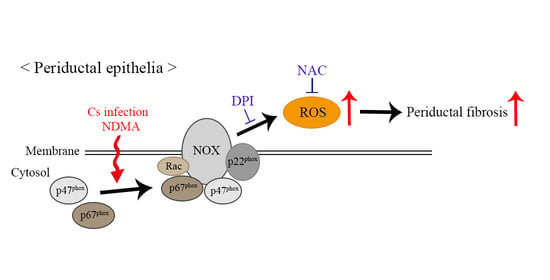
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of C. sinensis ESPs
2.3. Cell Culture and ESPs/NDMA Treatment
2.4. Ethics Statement
2.5. Preparation of C. sinensis Metacercariae and Infection
2.6. Subcellular Fractionation
2.7. Immunoblot Analysis
2.8. Detection of Intracellular ROS
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Activation of NOX and Increased Expression of Fibrosis-Related Proteins in ESPs-, NDMA-, or ESPs plus NDMA-Treated H69 Cells
3.2. Inhibitory Effect of NOX Inactivation on the Expression of Fibrosis-Related Proteins in ESPs-, NDMA-, or ESPs plus NDMA-Treated H69 Cells
3.3. Effect of ROS Scavenger on NOX Activation and of Fibrosis-Related Protein Expression
3.4. Expression of NOX Subunits (p47phox and p67phox) in Mouse Livers Infected with C. sinensis or/and NDMA Uptake
3.5. Collagen and Fibronectin Expression in Mouse Livers Infected with C. sinensis or/and NDMA Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jadeja, R.N.; Devkar, R.V.; Nammi, S. Oxidative Stress in Liver Diseases: Pathogenesis, Prevention, and Therapeutics. Oxid. Med. Cell Longev. 2017, 2017, 8341286. [Google Scholar] [CrossRef]
- Crosas-Molist, E.; Fabregat, I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 2015, 6, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.S.; Choi, D.; Choi, M.H.; Ji, Z.; Li, Z.; Cho, S.Y.; Hong, K.S.; Rim, H.J.; Hong, S.T. Correlation between sonographic findings and infection intensity in clonorchiasis. Am. J. Trop. Med. Hyg. 2005, 73, 1139–1144. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.S.; Pak, J.H.; Kim, J.B.; Bahk, Y.Y. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: A brief review. BMB Rep. 2016, 49, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Na, B.K.; Pak, J.H.; Hong, S.J. Clonorchis sinensis and clonorchiasis. Acta Trop. 2020, 203, 105309. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini—Multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yongvanit, P.; Pinlaor, S.; Bartsch, H. Oxidative and nitrative DNA damage: Key events in opisthorchiasis-induced carcinogenesis. Parasitol. Int. 2012, 61, 130–135. [Google Scholar] [CrossRef]
- Thamavit, W.; Bhamarapravati, N.; Sahaphong, S.; Vajrasthira, S.; Angsubhakorn, S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978, 38, 4634–4639. [Google Scholar]
- Lee, J.H.; Yang, H.M.; Bak, U.B.; Rim, H.J. Promoting role of Clonorchis sinensis infection on induction of cholangiocarcinoma during two-step carcinogenesis. Korean J. Parasitol. 1994, 32, 13–18. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 373, 96. [Google Scholar] [CrossRef]
- Maeng, S.; Lee, H.W.; Bashir, Q.; Kim, T.I.; Hong, S.J.; Lee, T.J.; Sohn, W.M.; Na, B.K.; Kim, T.S.; Pak, J.H. Oxidative stress-mediated mouse liver lesions caused by Clonorchis sinensis infection. Int. J. Parasitol. 2016, 46, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.H.; Choi, M.H.; Kim, W.H.; Jang, J.J.; Hong, S.T. Involvement of PSMD10, CDK4, and Tumor Suppressors in Development of Intrahepatic Cholangiocarcinoma of Syrian Golden Hamsters Induced by Clonorchis sinensis and N-Nitrosodimethylamine. PLoS Negl. Trop. Dis. 2015, 9, e0004008. [Google Scholar] [CrossRef]
- Prakobwong, S.; Pinlaor, S.; Yongvanit, P.; Sithithaworn, P.; Pairojkul, C.; Hiraku, Y. Time profiles of the expression of metalloproteinases, tissue inhibitors of metalloproteases, cytokines and collagens in hamsters infected with Opisthorchis viverrini with special reference to peribiliary fibrosis and liver injury. Int. J. Parasitol. 2009, 39, 825–835. [Google Scholar] [CrossRef]
- De Minicis, S.; Brenner, D.A. NOX in liver fibrosis. Arch. Biochem. Biophys. 2007, 462, 266–272. [Google Scholar] [CrossRef]
- Paik, Y.H.; Iwaisako, K.; Seki, E.; Inokuchi, S.; Schnabl, B.; Osterreicher, C.H.; Kisseleva, T.; Brenner, D.A. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology 2011, 53, 1730–1741. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.H.; Moon, J.H.; Kim, I.K.; Lee, M.R.; Hong, S.J.; Ahn, J.H.; Chung, J.W.; Pak, J.H. Free radicals enzymatically triggered by Clonorchis sinensis excretory-secretory products cause NF-kappaB-mediated inflammation in human cholangiocarcinoma cells. Int. J. Parasitol. 2012, 42, 103–113. [Google Scholar] [CrossRef]
- Pak, J.H.; Son, W.C.; Seo, S.B.; Hong, S.J.; Sohn, W.M.; Na, B.K.; Kim, T.S. Peroxiredoxin 6 expression is inversely correlated with nuclear factor-kappaB activation during Clonorchis sinensis infestation. Free Radic. Biol. Med. 2016, 99, 273–285. [Google Scholar] [CrossRef]
- Grubman, S.A.; Perrone, R.D.; Lee, D.W.; Murray, S.L.; Rogers, L.C.; Wolkoff, L.I.; Mulberg, A.E.; Cherington, V.; Jefferson, D.M. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am. J. Physiol. 1994, 266, G1060–G1070. [Google Scholar] [CrossRef]
- Vázquez-Medina, J.P.; Dodia, C.; Weng, L.; Mesaros, C.; Blair, I.A.; Feinstein, S.I.; Chatterjee, S.; Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6 modulates NADPH oxidase 2 activation via lysophosphatidic acid receptor signaling in the pulmonary endothelium and alveolar macrophages. FASEB J. 2016, 30, 2885–2898. [Google Scholar] [CrossRef] [Green Version]
- de Mochel, N.S.; Seronello, S.; Wang, S.H.; Ito, C.; Zheng, J.X.; Liang, T.J.; Lambeth, J.D.; Choi, J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology 2010, 52, 47–59. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Tsuchishima, M.; Tsutsumi, M. Molecular mechanisms in the pathogenesis of N-nitrosodimethylamine induced hepatic fibrosis. Cell Death Dis. 2019, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, G.E.; Adaramoye, O.A. Betulinic acid protects against N-nitrosodimethylamine-induced redox imbalance in testes of rats. Redox Rep. 2017, 22, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manea, A.; Tanase, L.I.; Raicu, M.; Simionescu, M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2010, 396, 901–907. [Google Scholar] [CrossRef]
- Cevik, M.O.; Katsuyama, M.; Kanda, S.; Kaneko, T.; Iwata, K.; Ibi, M.; Matsuno, K.; Kakehi, T.; Cui, W.; Sasaki, M.; et al. The AP-1 site is essential for the promoter activity of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme: Possible involvement of the ERK1/2-JunB pathway. Biochem. Biophys. Res. Commun. 2008, 374, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Maitra, U.; Singh, N.; Gan, L.; Ringwood, L.; Li, L. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J. Biol. Chem. 2009, 284, 35403–35411. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.M.; Bae, Y.M.; Choi, M.H.; Hong, S.T. Connexin 43 plays an important role in the transformation of cholangiocytes with Clonochis sinensis excretory-secretory protein and N-nitrosodimethylamine. PLoS Negl. Trop. Dis. 2019, 13, e0006843. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.M.; Kim, J.S.; Choi, M.H.; Hong, S.T.; Bae, Y.M. Effects of excretory/secretory products from Clonorchis sinensis and the carcinogen dimethylnitrosamine on the proliferation and cell cycle modulation of human epithelial HEK293T cells. Korean J. Parasitol. 2008, 46, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Kisseleva, T.; Brenner, D.A. The Role of NADPH Oxidases (NOXs) in Liver Fibrosis and the Activation of Myofibroblasts. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Valle, V.; Chávez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Yan, C.; Wang, L.; Li, B.; Zhang, B.B.; Zhang, B.; Wang, Y.H.; Li, X.Y.; Chen, J.X.; Tang, R.X.; Zheng, K.Y. The expression dynamics of transforming growth factor-beta/Smad signaling in the liver fibrosis experimentally caused by Clonorchis sinensis. Parasites Vectors 2015, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Pak, J.H.; Bashir, Q.; Kim, I.K.; Hong, S.J.; Maeng, S.; Bahk, Y.Y.; Kim, T.S. Clonorchis sinensis excretory-secretory products promote the migration and invasion of cholangiocarcinoma cells by activating the integrin β4-FAK/Src signaling pathway. Mol. Biochem. Parasitol. 2017, 214, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ryhanen, L.; Stenback, F.; Ala-Kokko, L.; Savolainen, E.R. The effect of malotilate on type III and type IV collagen, laminin and fibronectin metabolism in dimethylnitrosamine-induced liver fibrosis in the rat. J. Hepatol. 1996, 24, 238–245. [Google Scholar] [CrossRef]
- Bataller, R.; Schwabe, R.F.; Choi, Y.H.; Yang, L.; Paik, Y.H.; Lindquist, J.; Qian, T.; Schoonhoven, R.; Hagedorn, C.H.; Lemasters, J.J.; et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J. Clin. Invest. 2003, 112, 1383–1394. [Google Scholar] [CrossRef]
- Kono, H.; Rusyn, I.; Uesugi, T.; Yamashina, S.; Connor, H.D.; Dikalova, A.; Mason, R.P.; Thurman, R.G. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1005–G1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.J.; Park, Y.Y.; Yoon, G.; Cho, H.; Lee, J.H. Co-treatment with hepatocyte growth factor and TGF-beta1 enhances migration of HaCaT cells through NADPH oxidase-dependent ROS generation. Exp. Mol. Med. 2010, 42, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosoki, H.; Stegemann, A.; Taha, M.; Schnittler, H.; Luger, T.A.; Schröder, K.; Distler, J.H.; Kerkhoff, C.; Böhm, M. Targeting of NADPH oxidase in vitro and in vivo suppresses fibroblast activation and experimental skin fibrosis. Exp. Derm. 2017, 26, 73–81. [Google Scholar] [CrossRef]
- Sun, Y.; Pu, L.Y.; Lu, L.; Wang, X.H.; Zhang, F.; Rao, J.H. N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World J. Gastroenterol. 2014, 20, 15289–15298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-Filho, G.; Ferreira, C.; Schwengber, A.; Marroni, C.; Zettler, C.; Marroni, N. Role of N-acetylcysteine on fibrosis and oxidative stress in cirrhotic rats. Arq. Gastroenterol. 2008, 45, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Sathish, P.; Paramasivan, V.; Palani, V.; Sivanesan, K. N-acetylcysteine attenuates dimethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2011, 654, 181–186. [Google Scholar] [CrossRef]
- Perina, E.A.; Ivanov, V.V.; Pershina, A.G.; Perekucha, N.A.; Dzyuman, A.N.; Kaminskii, I.P.; Saltykova, I.V.; Sazonov, A.E.; Ogorodova, L.M. Imbalance in the glutathione system in Opisthorchis felineus infected liver promotes hepatic fibrosis. Acta Trop. 2019, 192, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Miao, N.J.; Xu, J.L.; Gan, X.X.; Xu, D.; Zhou, L.; Xue, H.; Zhang, W.; Lu, L.M. N-acetylcysteine alleviates angiotensin II-mediated renal fibrosis in mouse obstructed kidneys. Acta Pharmacol. Sin. 2016, 37, 637–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machicado, C.; Machicado, J.D.; Maco, V.; Terashima, A.; Marcos, L.A. Association of Fasciola hepatica Infection with Liver Fibrosis, Cirrhosis, and Cancer: A Systematic Review. PLoS Negl. Trop. Dis. 2016, 10, e0004962. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.H.; Li, S.; Jin, Y.; Choi, M.H.; Jang, J.J.; Hong, S.T. C3H/He Mice as an Incompatible Cholangiocarcinoma Model by Clonorchis sinensis, Dicyclanil and N-Nitrosodimethylamine. Korean J. Parasitol. 2016, 54, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Qiao, T.; Ma, R.H.; Luo, X.B.; Luo, Z.L.; Zheng, P.M. Cholecystolithiasis is associated with Clonorchis sinensis infection. PLoS ONE 2012, 7, e42471. [Google Scholar] [CrossRef] [Green Version]
- Altrock, E.; Sens, C.; Wuerfel, C.; Vasel, M.; Kawelke, N.; Dooley, S.; Sottile, J.; Nakchbandi, I.A. Inhibition of fibronectin deposition improves experimental liver fibrosis. J. Hepatol. 2015, 62, 625–633. [Google Scholar] [CrossRef]
- Liu, X.Y.; Liu, R.X.; Hou, F.; Cui, L.J.; Li, C.Y.; Chi, C.; Yi, E.; Wen, Y.; Yin, C.H. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol. Med. Rep. 2016, 14, 3669–3675. [Google Scholar] [CrossRef] [Green Version]
- Intuyod, K.; Priprem, A.; Limphirat, W.; Charoensuk, L.; Pinlaor, P.; Pairojkul, C.; Lertrat, K.; Pinlaor, S. Anti-inflammatory and anti-periductal fibrosis effects of an anthocyanin complex in Opisthorchis viverrini-infected hamsters. Food Chem. Toxicol. 2014, 74, 206–215. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.H.; Yi, J.; Hwang, M.K.; Hong, S.-J.; Sohn, W.-M.; Kim, T.-S.; Pak, J.H. The Overactivation of NADPH Oxidase during Clonorchis sinensis Infection and the Exposure to N-Nitroso Compounds Promote Periductal Fibrosis. Antioxidants 2021, 10, 869. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060869
Jeong JH, Yi J, Hwang MK, Hong S-J, Sohn W-M, Kim T-S, Pak JH. The Overactivation of NADPH Oxidase during Clonorchis sinensis Infection and the Exposure to N-Nitroso Compounds Promote Periductal Fibrosis. Antioxidants. 2021; 10(6):869. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060869
Chicago/Turabian StyleJeong, Ji Hoon, Junyeong Yi, Myung Ki Hwang, Sung-Jong Hong, Woon-Mok Sohn, Tong-Soo Kim, and Jhang Ho Pak. 2021. "The Overactivation of NADPH Oxidase during Clonorchis sinensis Infection and the Exposure to N-Nitroso Compounds Promote Periductal Fibrosis" Antioxidants 10, no. 6: 869. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10060869