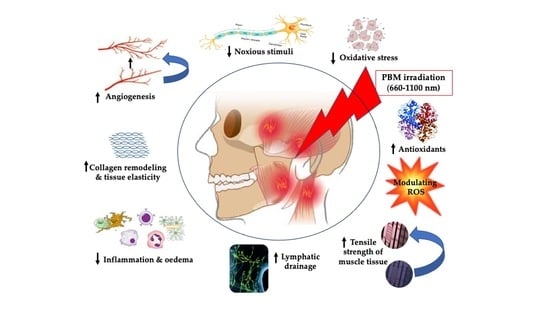
Role of Photobiomodulation Therapy in Modulating Oxidative Stress in Temporomandibular Disorders. A Systematic Review and Meta-Analysis of Human Randomised Controlled Trials
Abstract
:1. Introduction
- To explore the basis of and extrapolate the reasons for the inconsistencies among the data.
- To evaluate the sensitivity of the results’ methods of assessment and obtain vigorous standardised methodology.
- To attempt to propose a preliminary empirical consensus of PBM laser and LED dosimetry and treatment protocols.
- To postulate extraoral (EO) and intraoral (IO) treatment strategies for TMD for future randomised clinical trial (RCT) studies.
- The pathogenesis and aetiology of temporomandibular disorder (TMD) are complex and not clearly understood.
- Oxidative stress and reactive oxygen species play a vital part in TMD pathogenesis and its progression.
- Photobiomodulation (PBM) therapy is an effective treatment modality, as mono-therapy of various light sources of single wavelength or in combination of two wavelengths, in improving chronic pain, functionality, anxiety/depression and, subsequently, quality of life in patients with TMD.
- This review, for the first time, addressed the standardisation of methodology and PBM protocols by proposing suggested recommendations, which can only be used to pave the roadmap for future extensive research in the management of TMD chronic symptoms.
2. Materials and Methods
2.1. Protocol and PROSPERO Registration
2.2. Population (P), Intervention (I), Comparison (C) and Outcomes (O)—PICO
- I: Effect of PBMT with light-emitting diodes (LEDs) or laser on TMD, including chronic pain, masticatory malfunction, anxiety/depression and quality of life (QoL).
- C: Placebo (sham PBM), pharmacological approach, cognitive approach, physiotherapy, conservative treatment modalities (occlusal splint), ultrasound, TENS, alpha lipoic acid, needle therapy or combined therapy (PBM and any standard care treatment).
- O: Pain intensity (PI) reduction, functional enhancement, anxiety/depression improvement or QoL improvement.
2.3. Focused Questions of Review Search
- Does PBM with laser or LEDs or combined therapies have superior effects compared to placebo or TMD standard care, or combined therapies (PBMT and standard care), in reducing pain intensity or improving patients’ functionality and psychological status, as well as QoL?
- Does combined laser PBM therapy of red and IR wavelengths provide synergistic effects compared to placebo?
- Is it possible to propose clinical guidance and recommendations of PBMT (LEDs and laser) for TMD management?
2.4. Search Strategy
2.5. Relevant Free Keywords and MeSH Terms
2.6. Eligibility Criteria
2.6.1. Inclusion Criteria
- Studies of in vivo human randomised controlled trials (RCTs) (split-mouth, parallel or prospective), comparing the efficacy of PBMT to any other standard care treatment modalities or combined therapies (PBM and one standard care treatment).
- Studies investigating the effects of PBMT on TMD symptoms; chronic pain for ≥3 months in TMJ or masticatory muscles, loss of movement or masticatory malfunction for at least 3 months were included.
- Light sources: laser or light-emitting diodes (LEDs) with no wavelength restrictions.
- Studies reporting at least one of the following parameters as an outcome variable: pain score, functionality score, qualify of life or immunological profile.
- Studies reporting any of the following outcomes: immediately after treatment, middle of treatment and end of treatment.
- Studies reporting any follow-up timepoint: short-term, >2 weeks and <2 months; intermediate-term, ranging between >2 months and <6 months; and long-term, >6 months.
- If multiple terms of outcomes were reported within one period, a period the closest to two weeks, one month, three months and six months for each follow-up timepoint respectively used.
- All timepoints assessments of additional outcomes (all): baseline, immediate post treatment, short-term, intermediate-term and long-term follow-up.
- No language restrictions for search strategy.
- No restrictions on the reported laser parameters.
- Subjects with one or more of the following symptoms: mandibular activities aggravate pain and functional disabilities, pain clicking, mandibular movements (MM) limitation or myogenous or arthrogenous TMJ pain.
- Data search was during the period 1 January 2005–31 January 2021.
2.6.2. Exclusion Criteria
- Studies utilised home or stellate ganglion or acupuncture PBM (laser or LEDs) approach.
- Other neuropathic orofacial pain conditions not related to TMD.
- Studies utilised pharmacotherapy as a primary outcome.
- Studies utilised a combined physiotherapeutic, pharmacotherapy and homeopathic measure.
- Physiological or systematic conditions contributing to the pain.
- Subjects with the following systemic diseases: cardiovascular, infection, inflammatory, neurological, metabolic, rheumatoid, osteoarthritis (changes in the fossa and condyle), autoimmune disorders.
- Subjects with mental illnesses which could affect the clinical picture of patients, cervical disc herniation, history of trauma, TMJ surgery, musculo-articular pathologies, history of facial trauma, TMJ disc or condyle erosion, fibromyalgia, removable denture, missing more than one tooth in each quadrant and major malocclusion (anterior open bite, maxillary unilateral lingual cross-bite and overjet greater than 6 mm).
- Subjects with active head and neck malignant tumours.
- Pregnant and lactating women.
- Subjects who underwent treatment for headache or bruxism in last 6 months prior to their enrollment in RCT study
- Studies utilised homeopathic therapy as a comparative therapy.
- Narrative and systematic reviews, case reports, in vitro studies, in vivo animal studies, commentaries, interviews, updates or case series.
- A necessity of initiating the use of any type of medications during any of phase of the study.
- Studies investigating acute TMD or acute versus (vs.) chronic TMD.
2.7. Types of Outcomes Measures
2.7.1. Primary Outcomes
2.7.2. Secondary Outcomes
- Functional improvement (muscles movements: mouth opening and closing and chewing) from baseline up to the end of follow-up.
- Reduction in anxiety/depression and improved QoL from baseline up to the end of follow-up.
2.8. Data Extraction
2.9. Qualitive Analysis
- Bias arising from the randomisation process.
- Bias due to deviations from intended interventions.
- Bias due to missing outcome data.
- Bias in measurement of the outcome.
- Bias in selection of the reported result.
2.10. Statistical Analysis of Data
3. Results
3.1. Study Selection
3.2. Characteristics of the Study Populations (Table S1)
3.2.1. Sample Size
3.2.2. Racial Background
3.2.3. Gender Distribution
3.2.4. Age Distribution
3.2.5. Presented Symptoms
3.2.6. Aetiology of TMD
3.2.7. Affected Area
3.2.8. Functionality Problems
3.3. Study Characteristics
3.3.1. Country of Origin
3.3.2. Study Design
3.3.3. Intervention Group
3.3.4. Documentation of Reported PBM Irradiation Parameters
Utilised Wavelength
Emission Mode
Pulse Width (s, μs) and Frequency (Hz)
Laser/LED Tip-Tissue Distance (Contact/Non-Contact)
Reported Energy (J)
Power Output and Therapeutic Power Output (W, mW)
Energy Density (Dose or Fluence, J/cm2)
Exposure Time (s)
Frequency of the Treatment (Number of Sessions)
Duration of the Treatment
Spot Size/Spot Area/Beam Diameter/Fibre-Tip Diameter Parameters
Methods of PBM Applications, and Number and Allocation of TP
3.3.5. Follow-Up Assessment
3.3.6. Assessment Methods
3.4. Qualitative Assessment
3.5. Impact Factor of the Published Papers
3.6. Quantitative Assessment
3.6.1. Outcome Variables
3.6.2. Subgroup Analysis
3.6.3. Sensitivity Analysis
3.6.4. Publication Bias
4. Discussion
4.1. Characteristics of the Reported Recruited Subjects (Population’s Phenotype)
4.2. Methodology Quality
4.2.1. Evaluation of Study Design
4.2.2. Diagnostic Criteria
- Despite RDC/TMD being the most common TMD diagnostic tool utilised by various researchers, there are three fundamental downsides to it, which are as follows [112]: (a) Relatively limited use due to the diversity of clinically presented TMD symptoms. Therefore, this tool can be utilised but many of the presented symptoms will not fit in one or any category. (b) They do not account for cervical spine involvement, which is crucial to thorough evaluation and management. (c) Various identified patients’ experiencing pain symptoms such as hyperalgesia and/or allodynia should be managed accordingly, despite the fact that these variables are not addressed by RDC/TMD criteria.
- Regardless of if TMD participants have symptoms in the cervical regions, a full clinical assessment is required and the cervical muscles’ TP need to be addressed in the irradiation protocol. This is due to an altered neuro-biomechanical function of the cervical spine, which can apply stress on the TMJ, causing TMD. There is evidence to support that the craniomandibular region and upper cervical spine are related from anatomical, biochemical and neurophysiological standpoints [113,114], due to a neuroanatomical link between the orofacial and cervical regions, as well changes in the isometric strength of cervical flexors, according to the bite position of TMD patients [115,116]. Hence, the manual muscle test (MMT) is a reliable and useful clinical diagnostic tool for cervical muscles assessment to be considered [117].
4.2.3. Assessment of the Outcome Measures
4.3. Role of Tissue Optical Properties in Determining the Therapeutic Dosimetry
4.4. Evaluation of EO (Transcutaneous) and IO (Transmucosal) PBM Therapy Approaches
4.5. Evaluation of Reported Laser Treatment Parameters
4.5.1. Utilisation of a Single Wavelength in Test Group
4.5.2. Utilisation Two Laser Wavelengths in Test Group
4.5.3. Mixed light Sources in the Test Groups
4.5.4. Evaluation of the Trigger and Palpable Points and Applications of Irradiation
4.6. Role of RoB Assessment
4.7. Role of Meta-Analysis Outcome
5. Conclusions and Future Direction
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harper, D.E.; Schrepf, A.; Clauw, D.J. Pain mechanisms and centralized pain in temporomandibular disorders. J. Dent. Res. 2016, 95, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Torre Canales, G.; Câmara-Souza, M.B.; Muñoz Lora, V.R.M.; Guarda-Nardini, L.; Conti, P.C.R.; Rodrigues Garcia, R.M.; Del Bel Cury, A.A.; Manfredini, D. Prevalence of psychosocial impairment in temporomandibular disorder patients: A systematic review. J. Oral Rehabil. 2018, 45, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Natu, V.P.; Yap, A.U.; Su, M.H.; Irfan Ali, N.M.; Ansari, A. Temporomandibular disorder symptoms and their association with quality of life, emotional states and sleep quality in South-East Asian youths. J. Oral Rehabil. 2018, 45, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Benoliel, R.; Svensson, P.; Evers, S.; Wang, S.J.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic secondary headache or orofacial pain. Pain 2019, 160, 60–68. [Google Scholar] [CrossRef]
- Sessle, B.J. The neural basis of temporomandibular joint and masticatory muscle pain. J. Orofac. Pain 1999, 13, 238–245. [Google Scholar]
- Fernández-de-las-Penas, C.; Svensson, P. Myofascial Temporomandibular Disorder. Curr. Rheumatol. Rev. 2016, 12, 40–54. [Google Scholar] [CrossRef]
- Fricton, J.; Look, J.O.; Wright, E. Systematic review and meta-analysis of randomized controlled trials evaluating intraoral orthopedic appliances for temporomandibular disorders. J. Orofac. Pain 2010, 24, 237–254. [Google Scholar]
- McNeely, M.L.; Armijo Olivo, S.; Magee, D.J. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys. Ther. 2006, 86, 710–725. [Google Scholar] [CrossRef] [Green Version]
- Mujakperuo, H.R.; Watson, M.; Morrison, R. Pharmacological interventions for pain in patients with temporomandibular disorders. Cochrane Database Syst. Rev. 2010, 10, CD004715. [Google Scholar] [CrossRef]
- List, T.; Axelsson, S. Management of TMD: Evidence from systematic reviews and meta-analyses. J. Oral Rehabil. 2010, 37, 430–435. [Google Scholar] [CrossRef]
- List, T.; Jensen, R.H. Temporomandibular disorders: Old ideas and new concepts. Cephalagia 2017, 37, 692–704. [Google Scholar] [CrossRef]
- Wade-Vallance, A.; Ng, M.; Kuo, K.; Luo, C.; Adams, S.; Dhaliwal, G.; Yu, C. A central role for reactive oxygen species (ROS) in the pathogenesis of temporomandibular joint disorders: All roads lead to ROS. Catal. Facet. Biochem. Biomed. Sci. 2017, 2, 1–7. [Google Scholar]
- Bouloux, G.F. The use of synovial fluid analysis for diagnosis of temporomandibular joint disorders. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 251–256. [Google Scholar] [CrossRef]
- Braz, M.A.; Freitas Portella, F.; Seehaber, K.A.; Bavaresco, C.S.; Rivaldo, E.G. Association between oxidative stress and temporomandibular joint dysfunction: A narrative review. J. Oral Rehabil. 2020, 47, 536–546. [Google Scholar] [CrossRef]
- Mirahmadi, F.; Koolstra, J.H.; Lobbezoo, F.; van Lenthe, G.H.; Ghazanfari, S.; Snabel, J.; Stoop, R.; Everts, V. Mechanical stiffness of TMJ condylar cartilage increases after artificial aging by ribose. Arch. Oral Biol. 2018, 87, 102–109. [Google Scholar] [CrossRef]
- Loreto, C.; Filetti, V.; Almeida, L.E.; La Rosa, G.; Leonardi, R.; Grippaudo, C.; Lo Giudice, A. MMP-7 and MMP-9 are overexpressed in the synovial tissue from severe temporomandibular joint dysfunction. Eur. J. Histochem. EJH 2020, 64, 3113. [Google Scholar] [CrossRef]
- Melis, M.; Di Giosia, M. The role of genetic factors in the etiology of temporomandibular disorders: A review. Cranio 2016, 34, 43–51. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Benedicenti, S.; Amaroli, A.; Saălaăgean, T.; Pop, I.D.; Todea, D.; Bordea, I.R. Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future. Cancers 2020, 12, 1949. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A comparative study between the effectiveness of 980nm photobiomodulation, delivered by Gaussian versus flattop profiles on osteoblasts maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Hanna, R.; Dalvi, S.; Amaroli, A.; De Angelis, N.; Benedicenti, S. Effects of photobiomodulation on bone defects grafted with bone substitutes: A systematic review of in vivo animal studies. J. Biophotonics 2021, e202000267. [Google Scholar] [CrossRef]
- Ferraresi, C.; Hamblin, M.R.; Parizotto, N.A. Low-level laser (light) therapy (LLLT) on muscle tissue: Performance, fatigue and repair benefited by the power of light. Photonics Lasers Med. 2012, 1, 267–286. [Google Scholar] [CrossRef] [Green Version]
- Salmos-Brito, J.A.L.; de Menezes, R.F.; Teixeira, C.E.C.; Gonzaga, R.K.; Rodrigues, B.H.; Braz, R.; Bessa-Nogueira, R.V.; Gerbi, M.E. Evaluation of low-level laser therapy in patients with acute and chronic temporomandibular disorders. Lasers Med. Sci. 2013, 28, 57–64. [Google Scholar] [CrossRef]
- Sakurai, Y.; Yamaguchi, M.; Abiko, Y. Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur. J. Oral Sci. 2000, 108, 29–34. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A.; Klimova, M.; Agranovich, I.; Terskov, A.; Shirokov, A.; Vinnik, V.; Kuzmina, A.; Lezhnev, N.; et al. Photobiomodulation of lymphatic drainage and clearance: Perspective strategy for augmentation of meningeal lymphatic functions. Biomed. Opt. Express 2020, 11, 725–734. [Google Scholar] [CrossRef]
- Cotler, H.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The use of low-level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop. Rheumatol. 2015, 2, 00068. [Google Scholar] [CrossRef]
- Mangueira, N.M.; Xavier, M.; de Souza, R.A.; Salgado, M.A.; Silveira, L., Jr.; Villaverde, A.B. Effect of low-level laser therapy in an experimental model of osteoarthritis in rats evaluated through Raman spectroscopy. Photomed. Laser Surg. 2015, 33, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomazoni, S.S.; Costa, L.D.C.M.; Guimarães, L.S.; Araujo, A.C.; Nascimento, D.P.; Medeiros, F.C.; Avanzi, M.A.; Costa, L.O.P. Effects of photobiomodulation therapy in patients with chronic non-specific low back pain: Protocol for a randomised placebo-controlled trial. BMJ Open 2017, 7, e017202. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.R.; Rigby, J.H.; Mettler, J.A.; McCurdy, K.W. The Effectiveness of Photobiomodulation Therapy Versus Cryotherapy for Skeletal Muscle Recovery: A critically Appraised Topic. J. Sport Rehabil. 2021, 28, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, A.; Sgolastra, F.; Gatto, R.; Mattei, A.; Monaco, A.J. Effectiveness of low-level laser therapy in temporomandibular disorders: A systematic review and meta-analysis. J. Orofac. Pain 2011, 25, 298–307. [Google Scholar]
- Maia, M.L.; Bonjardim, L.R.; Quintans Jde, S.; Ribeiro, M.A.; Maia, L.G.; Conti, P.C. Effect of low-level laser therapy on pain levels in patients with temporomandibular disorders: A systematic review. J. Appl. Oral Sci. 2012, 20, 594–602. [Google Scholar] [CrossRef]
- Junér, J.; Hosseinpour, S.; Fekrazad, R. Photobiomodulation in Temporomandibular Disorders. Photobiomodul. Photomed. Laser Surg. 2019, 37, 826–836. [Google Scholar] [CrossRef]
- Kim, W.S.; Calderhead, R.G. Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Ther. 2011, 20, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Herpich, C.M.; Leal-Junior, E.C.P.; Gomes, C.A.F.; Gloria, I.P.D.S.; Amaral, A.P.; Amaral, M.F.R.S.; Politti, F.; Biasotto-Gonzalez, D.A. Immediate and short-term effects of phototherapy on pain, muscle activity, and joint mobility in women with temporomandibular disorder: A randomized, double-blind, placebo-controlled, clinical trial. Disabil. Rehabil. 2018, 40, 2318–2324. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 The Cochrane Collaboration. 2011. Available online: http://www.cochrane-handbook.org (accessed on 28 May 2021).
- Dworkin, S.F. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. J. Craniomandibular Disord. 1992, 6, 301–355. [Google Scholar]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- McHugh, M.L. Inter-rate reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Altman, D.G.; Schulz, K.F.; Moher, D.; Egger, M.; Davidoff, F.; Elbourne, D.; Gøtzsche, P.C.; Lang, T.; CONSORT GROUP (Consolidated Standards of Reporting Trials). The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann. Intern Med. 2001, 134, 663–694. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Eldridge, S.; Li, T. Chapter 23: Including variants on randomized trials. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Cochrane, AB, Canada, 2019; Available online: www.training.cochrane.org/handbook (accessed on 23 June 2021).
- The Cochrane Collaboration. Review Manager (RevMan) [Computer Program]. Version 5.4.1; The Cochrane Collaboration: London, UK, 2020. [Google Scholar]
- Lau, J.; Ioannidis, J.P.; Schmid, C.H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 1997, 127, 820–826. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.R.; Barros, R.Q.; Gonçalves, A.S.; Freitas, P.M. Photobiomodulation therapy on the palliative care of temporomandibular disorder and orofacial/cervical skull pain: Study protocol for a randomized controlled clinical trial. Trials 2019, 20, 200. [Google Scholar] [CrossRef]
- Sousa, D.F.M.; Gonçalves, M.L.L.; Politti, F.; Lovisetto, R.D.P.; Fernandes, K.P.S.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Photobiomodulation with simultaneous use of red and infrared light emitting diodes in the treatment of temporomandibular disorder: Study protocol for a randomized, controlled and double-blind clinical trial. Medicine 2019, 98, e14391. [Google Scholar] [CrossRef] [PubMed]
- Leal de Godoy, C.H.; Motta, L.J.; Garcia, E.J.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Sfalcin, R.A.; Motta, P.B.; Politti, F.; Bussadori, S.K. Electromyographic evaluation of a low-level laser protocol for the treatment of temporomandibular disorder: A randomized, controlled, blind trial. J. Phys. Ther. Sci. 2017, 29, 2107–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.A.; de Oliveira, R.G.; Guimarães, J.P.; Carvalho, A.C.; De Paula, M.V. Laser acupuncture in patients with temporomandibular dysfunction: A randomized controlled trial. Lasers Med. Sci. 2013, 28, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Kulekcioglu, S.; Sivrioglu, K.; Ozcan, O.; Parlak, M. Effectiveness of low-level laser therapy in temporomandibular disorder. Scand. J. Rheumatol. 2003, 32, 114–118. [Google Scholar] [CrossRef]
- Madani, S.; Ahrari, F.; Nasiri, F.; Abtahi, M.; Tuner, J. Low-level laser therapy for management of TMJ osteoarthritis. Cranio 2014, 32, 38–44. [Google Scholar] [CrossRef]
- Cavalcanti, M.F.; Silva, U.H.; Leal-Junior, E.C.; Lopes-Martins, R.A.; Marcos, R.L.; Pallotta, R.C.; Diomede, F.; Trubiani, O.; De Isla, N.; Frigo, L. Comparative study of the physiotherapeutic and drug protocol and low-level laser irradiation in the treatment of pain associated with temporomandibular dysfunction. Photomed. Laser Surg. 2016, 34, 652–656. [Google Scholar] [CrossRef]
- Venancio Rde, A.; Camparis, C.M.; Lizarelli Rde, F. Low intensity laser therapy in the treatment of temporomandibular disorders: A double-blind study. J. Oral Rehabil. 2005, 32, 800–807. [Google Scholar] [CrossRef]
- Çetiner, S.; Kahraman, S.A.; Yücetaş, S. Evaluation of low-level laser therapy in the treatment of temporomandibular disorders. Photomed. Laser Surg. 2006, 24, 637–641. [Google Scholar] [CrossRef]
- Fikácková, H.; Dostálová, T.; Navrátil, L.; Klaschka, J. Effectiveness of low-level laser therapy in temporomandibular joint disorders: A placebo-controlled study. Photomed. Laser Surg. 2007, 25, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Mazzetto, M.O.; Carrasco, T.G.; Bidinelo, E.F.; de Andrade Pizzo, R.C.; Mazzetto, R.G. Low intensity laser application in temporomandibular disorders: A phase I double-blind study. Cranio 2007, 25, 186–192. [Google Scholar] [CrossRef]
- Frare, J.C.; Nicolau, R.A. Clinical analysis of the effect of laser photobiomodulation (GaAs—904 nm) on temporomandibular joint dysfunction. Rev. Bras. Fisioter. 2008, 12, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, L.A.; Firoozmand, L.M.; da Silva, A.P.; Camargo, S.E.; Oliveira, W. Efficacy of low-level laser therapy in the treatment of temporomandibular disorder. Int. Dent. J. 2008, 58, 213–217. [Google Scholar] [CrossRef]
- Lassemi, E.; Jafari, S.M.; Motamedi, M.H.K.; Navi, F.; Lasemi, R. Low- level laser therapy in the management of temporamandibular joint disorder. J. Oral Laser Appl. 2008, 8, 83–86. [Google Scholar]
- Carrasco, T.G.; Mazzetto, M.O.; Mazzetto, R.G.; Mestriner, W., Jr. Low intensity laser therapy in temporomandibular disorder: A phase II double-blind study. Cranio 2008, 26, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Emshoff, R.; Bösch, R.; Pümpel, E.; Schöning, H.; Strobl, H. Low-level laser therapy for treatment of temporomandibular joint pain: A double-blind and placebo-controlled trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, T.G.; Guerisoli, L.D.; Guerisoli, D.M.; Mazzetto, M.O. Evaluation of low intensity laser therapy in myofascial pain syndrome. Cranio 2009, 27, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Shirani, A.M.; Gutknecht, N.; Taghizadeh, M.; Mir, M. Low-level laser therapy and myofacial pain dysfunction syndrome: A randomized controlled clinical trial. Lasers Med. Sci. 2009, 24, 715–720. [Google Scholar] [CrossRef]
- Venezian, G.C.; da Silva, M.A.; Mazzetto, R.G.; Mazzetto, M.O. Low level laser effects on pain to palpation and electromyographic activity in TMD patients: A double-blind, randomized, placebo-controlled study. Cranio 2010, 28, 84–91. [Google Scholar] [CrossRef]
- Öz, S.; Gökçen-Röhlig, B.; Saruhanoglu, A.; Tuncer, E.B. Management of myofascial pain: Low-level laser therapy versus occlusal splints. J. Craniofac. Surg. 2010, 21, 1722–1728. [Google Scholar] [CrossRef] [Green Version]
- Marini, I.; Gatto, M.R.; Bonetti, G.A. Effects of superpulsed low-level laser therapy on temporomandibular joint pain. Clin. J. Pain 2010, 26, 611–616. [Google Scholar] [CrossRef]
- Rohlig, B.G.; Kipirdi, S.; Meric, U.; Capan, N.; Keskin, H. Masticatory muscle pain and low-level laser therapy: A double-blind and placebo-controlled study. Turk. J. Phys. Med. Rehabil. Turk. Fiz. Tip Rehabil. Derg. 2011, 57, 31–37. [Google Scholar] [CrossRef]
- Sattayut, S.; Bradley, P. A study of the influence of low intensity laser therapy on painful temporomandibular disorder patients. Laser Ther. 2012, 21, 183–192. [Google Scholar] [CrossRef] [Green Version]
- de Carli, M.L.; Guerra, M.B.; Nunes, T.B.; di Matteo, R.C.; de Luca, C.E.; Aranha, A.C.; Bolzan, M.C.; Witzel, A.L. Piroxicam and laser phototherapy in the treatment of TMJ arthralgia: A double-blind randomised controlled trial. J. Oral Rehabil. 2013, 40, 171–178. [Google Scholar] [CrossRef]
- da Silva, M.A.; Botelho, A.L.; Turim, C.V.; da Silva, A.M. Low level laser therapy as an adjunctive technique in the management of temporomandibular disorders. Cranio 2012, 30, 264–271. [Google Scholar] [CrossRef]
- Panhoca, V.H.; Lizarelli, R.D.F.Z.; Nunez, S.C.; de Andrade Pizzo, R.C.; Grecco, C.; Paolillo, F.R.; Bagnato, V.S. Comparative clinical study of light analgesic effect on temporomandibular disorder (TMD) using red and infrared led therapy. Lasers Med. Sci. 2015, 30, 815–822. [Google Scholar] [CrossRef]
- Uemoto, L.; Garcia, M.A.; Gouvêa, C.V.; Vilella, O.V.; Alfaya, T.A. Laser therapy and needling in myofascial trigger point deactivation. J. Oral Sci. 2013, 55, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Ahrari, F.; Madani, A.S.; Ghafouri, Z.S.; Tunér, J. The efficacy of low-level laser therapy for the treatment of myogenous temporomandibular joint disorder. Lasers Med. Sci. 2014, 29, 551–557. [Google Scholar] [CrossRef]
- Demirkol, N.; Sari, F.; Bulbul, M.; Demirkol, M.; Simsek, I.; Usumez, A. Effectiveness of occlusal splints and low-level laser therapy on myofascial pain. Lasers Med. Sci. 2015, 30, 1007–1012. [Google Scholar] [CrossRef]
- Pereira, T.S.; Flecha, O.D.; Guimarães, R.C.; de Oliveira, D.; Botelho, A.M.; Ramos Glória, J.C.; Aguiar Tavano, K.T. Efficacy of red and infrared lasers in treatment of temporomandibular disorders-A double-blind, randomized, parallel clinical trial. Cranio 2014, 32, 51–56. [Google Scholar] [CrossRef]
- De Moraes Maia, M.L.; Ribeiro, M.A.; Maia, L.G.; Stuginski-Barbosa, J.; Costa, Y.M.; Porporatti, A.L.; Conti, P.C.; Bonjardim, L.R. Evaluation of low-level laser therapy effectiveness on the pain and masticatory performance of patients with myofascial pain. Lasers Med. Sci. 2014, 29, 29–35. [Google Scholar] [CrossRef]
- Sancakli, E.; Gökçen-Röhlıg, B.; Balık, A.; Öngül, D.; Kıpırdı, S.; Keskın, H. Early results of low-level laser application for masticatory muscle pain: A double-blind randomized clinical study. BMC Oral Health 2015, 15, 131. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, D.W.; Lages, F.S.; Guimarães, R.C.; Pereira, T.S.; Botelho, A.M.; Glória, J.C.R.; Tavano, K.T.A.; Gonçalves, P.F.; Flecha, O.D. Do TMJ symptoms improve and last across time after treatment with red (660 nm) and infrared (790 nm) low level laser treatment (LLLT)? A survival analysis. Cranio 2017, 35, 372–378. [Google Scholar] [CrossRef]
- Costa, S.A.P.; Florezi, G.P.; Artes, G.E.; Costa, J.R.D.; Gallo, R.T.; Freitas, P.M.; Witzel, A.L. The analgesic effect of photobiomodulation therapy (830 nm) on the masticatory muscles: A randomized, double-blind study. Braz. Oral Res. 2017, 31, e107. [Google Scholar] [CrossRef] [Green Version]
- Seifi, M.; Ebadifar, A.; Kabiri, S.; Badiee, M.R.; Abdolazimi, Z.; Amdjadi, P. Comparative effectiveness of Low Level Laser therapy and Transcutaneous Electric Nerve Stimulation on Temporomandibular Joint Disorders. J. Lasers Med. Sci. 2017, 8, S27–S31. [Google Scholar] [CrossRef] [Green Version]
- Shobha, R.; Narayanan, V.S.; Jagadish Pai, B.S.; Jaishankar, H.P.; Jijin, M.J. Low-level laser therapy: A novel therapeutic approach to temporomandibular disorder—A randomized, double-blinded, placebo-controlled trial. Indian J. Dent. Res. 2017, 28, 380–387. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Hajian, K.; Shahidi, S.; Piroozi, S. Comparison of the Effects of Transcutaneous Electrical Nerve Stimulation and Low-Level Laser Therapy on Drug-Resistant Temporomandibular Disorders. J. Dent. 2017, 18, 187–192. [Google Scholar]
- Varma, S.R.; al Shayeb, M.; el Kaseh, A.; Kuduruthullah, S.; Ashekhi, A.; al Khader, E. Effectiveness of low-level laser therapy in the Management of the Temporomandibular Joint Disorders: A Placebo-controlled Trial. World J. Dent. 2018, 9, 316–320. [Google Scholar] [CrossRef]
- Borges, R.M.M.; Cardoso, D.S.; Flores, B.C.; da Luz, R.D.; Machado, C.R.; Cerveira, G.P.; Daitx, R.B.; Dohnert, M.B. Effects of different photobiomodulation dosimetries on temporomandibular dysfunction: A randomized, double-blind, placebo-controlled clinical trial. Lasers Med. Sci. 2018, 33, 1859–1866. [Google Scholar] [CrossRef]
- Brochado, F.T.; Jesus, L.H.; Carrard, V.C.; Freddo, A.L.; Chaves, K.D.; Martins, M.D. Comparative effectiveness of photobiomodulation and manual therapy alone or combined in TMD patients: A randomized clinical trial. Braz. Oral Res. 2018, 32, e50. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.A.; Melchior, M.O.; Valencise Magri, L.; Mazzetto, M.O. Can the severity of orofacial myofunctional conditions interfere with the response of analgesia promoted by active or placebo low-level laser therapy? Cranio 2020, 38, 240–247. [Google Scholar] [CrossRef]
- Peimani, A.; Keshavarz, S.; Fathollahi, M.S. Comparison of Low-Level Laser Therapy and Drug Therapy in Patients with Temporomandibular Disorders: A Randomized Clinical Trial. J. Oral Health Dent. 2020, 38, 240–247. [Google Scholar] [CrossRef]
- Nadershah, M.; Abdel-Alim, H.M.; Bayoumi, A.M.; Jan, A.M.; Elatrouni, A.; Jadu, F.M. Photobiomodulation Therapy for Myofascial Pain in Temporomandibular Joint Dysfunction: A Double-Blinded Randomized Clinical Trial. J. Maxillofac. Oral Surg. 2020, 19, 93–97. [Google Scholar] [CrossRef]
- Magri, L.V.; Bataglion, C.; Leite-Panissi, C.R.A. Follow-up results of a randomized clinical trial for low-level laser therapy in painful TMD of muscular origins. Cranio 2019, 1–8. [Google Scholar] [CrossRef]
- Al-Quisi, A.F.; Al-Anee, A.M.; Al-Jumaily, H.A.; Bahr, E.F.; Finjan, D.A. Efficacy of the LED Red Light Therapy in the Treatment of Temporomandibular Disorders: Double Blind Randomized Controlled Trial. Pain Res. Treat. 2019, 2019, 8578703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herpich, C.M.; Leal-Junior, E.C.P.; Politti, F.; de Paula Gomes, C.A.F.; Dos Santos Glória, I.P.; de Souza Amaral, M.F.R.; Herpich, G.; de Azevedo, L.M.A.; de Oliveira Gonzalez, T.; Biasotto-Gonzalez, D.A. Intraoral photobiomodulation diminishes pain and improves functioning in women with temporomandibular disorder: A randomized, sham-controlled, double-blind clinical trial: Intraoral photobiomodulation diminishes pain in women with temporomandibular disorder. Lasers Med. Sci. 2020, 35, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.; Bhate, K.; Santish Kumar, S.N.; Kshirsagar, K.; Jagtap, B.; Kakodkar, P. Comparative evaluation of low-level laser therapy and ultrasound heat therapy in reducing temporomandibular joint disorder pain. J. Dent. Anesth. Pain Med. 2019, 19, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Sobral, A.P.T.; Godoy, C.L.H.; Fernandes, K.P.S.; Bussadori, S.K.; Ferrari, R.A.M.; Horliana, A.C.R.T.; Monken, S.F.; Motta, L.J. Photomodulation in the treatment of chronic pain in patients with temporomandibular disorder: Protocol for cost-effectiveness analysis. BMJ Open 2018, 8, e018326. [Google Scholar] [CrossRef]
- Maracci, L.M.; Stasiak, G.; de Oliveira Chami, V.; Franciscatto, G.J.; Milanesi, J.; Figueiró, C.; Bernardon Silva, T.; Guimarães, M.B.; Marquezan, M. Treatment of myofascial pain with a rapid laser therapy protocol compared to occlusal splint: A double-blind, randomized clinical trial. Cranio 2020, 1–7. [Google Scholar] [CrossRef]
- Chellappa, D.; Thirupathy, M. Comparative efficacy of low-Level laser and TENS in the symptomatic relief of temporomandibular joint disorders: A randomized clinical trial. Indian J. Dent. Res. 2020, 31, 42–47. [Google Scholar] [CrossRef]
- Monteiro, L.; Ferreira, R.; Resende, T.; Pacheco, J.J.; Salazar, F. Effectiveness of Photobiomodulation in Temporomandibular Disorder-Related Pain Using a 635 nm Diode Laser: A Randomized, Blinded, and Placebo-Controlled Clinical Trial. Photobiomodul. Photomed. Laser Surg. 2020, 38, 280–288. [Google Scholar] [CrossRef]
- Bueno, C.H.; Pereira, D.D.; Pattussi, M.P.; Grossi, P.K.; Grossi, M.L. Gender differences in temporomandibular disorders in adult populational studies: A systematic review and meta-analysis. J. Oral Rehabil. 2018, 45, 720–729. [Google Scholar] [CrossRef]
- Vilanova, L.S.R.; Gonçalves, T.M.; Meirelles, L.; Garcia, R.C. Hormonal fluctuations intensify temporomandibular disorder pain without impairing masticatory function. Int. J. Prosthodont. 2015, 28, 72–74. [Google Scholar] [CrossRef] [Green Version]
- Al-Harthy, M.; Ohrbach, R.; Michelotti, A.; List, T. The effect of culture on pain sensitivity. J. Oral Rehabil. 2016, 43, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Theorell, T.; Hammarström, A.; Aronsson, G.; Träskman Bendz, L.; Grape, T.; Hogstedt, C.; Marteinsdottir, I.; Skoog, I.; Hall, C. A systematic review including meta-analysis of work environment and depressive symptoms. BMC 2015, 15, 738. [Google Scholar] [CrossRef] [Green Version]
- Racine, M.; Tousignant-Laflamme, Y.; Kloda, L.A.; Dion, D.; Dupuis, G.; Choinieère, M. A systematic literature review of 10 years of re-search on sex/gender and experimental pain perception-part 1: Are there really differences between women and men? Pain 2012, 153, 602–618. [Google Scholar] [CrossRef]
- Racine, M.; Tousignant-Laflamme, Y.; Kloda, L.A.; Dion, D.; Dupuis, G.; Choinière, M. A systematic literature review of 10 years of research on sex/gender and pain perception—Part 2: Do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 2012, 153, 619–635. [Google Scholar] [CrossRef]
- Seidenari, S.; Pagnoni, A.; Di Nardo, A.; Giannetti, A. Echographic evaluation with image analysis of normal skin: Variations according to age and sex. Skin Pharmacol. 1994, 7, 201–209. [Google Scholar] [CrossRef]
- Gambichler, T.; Matip, R.; Moussa, G.; Altmeyer, P.; Hoffmann, K. In vivo data of epidermal thickness evaluated by optical coherence tomography: Effects of age, gender, skin type, and anatomic site. J. Dermatol. Sci. 2006, 44, 145–152. [Google Scholar] [CrossRef]
- Shuster, S.; Black, M.M.; McVitie, E. The influence of age and sex on skin thickness, skin collagen and density. Br. J. Dermatol. 1975, 93, 639–643. [Google Scholar] [CrossRef]
- Sabino, C.P.; Deana, A.M.; Yoshimura, T.M.; da Silva, D.F.; França, C.M.; Hamblin, M.R.; Ribeiro, M.S. The optical properties of mouse skin in the visible and near infrared spectral regions. J. Photochem. Photobiol. B 2016, 160, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, T.; Yamaguchi, Y.; Batzer, J.; Coelho, S.G.; Zmudzka, B.Z.; Miller, S.A.; Wolber, R.; Beer, J.Z.; Hearing, V.J. Mechanisms of skin tanning in different racial/ ethnic groups in response to ultraviolet radiation. J. Investig. Dermatol. 2005, 124, 1326–1332. [Google Scholar] [CrossRef] [Green Version]
- Vilanova, L.S.; Garcia, R.C.; List, T.; Alstergren, P. Diagnostic criteria for temporomandibular disorders: Self-instruction or formal training and calibration? J. Headache Pain 2015, 16, 505. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, P.O.; Häggman-Henrikson, B.; Nordh, E.; Zafar, H. Co-ordinated mandibular and head-neck movements during rhythmic jaw activities in man. J. Dent. Res. 2000, 79, 1378–1384. [Google Scholar] [CrossRef]
- Cuenca-Martínez, F.; Herranz-Gómez, A.; Madroñero-Miguel, B.; Reina-Varona, Á.; La Touche, R.; Angulo-Díaz-Parreño, S.; Pardo-Montero, J.; Del Corral, T.; López-de-Uralde-Villanueva, I. Craniocervical and Cervical Spine Features of Patients with Temporomandibular Disorders: A Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Med. 2020, 9, 2806. [Google Scholar] [CrossRef]
- Giannakopoulos, N.N.; Schindler, H.J.; Rammelsberg, P.; Eberhard, L.; Schmitter, M.; Hellmann, D. Co-activation of jaw and neck muscles during submaximum clenching in the supine position. Arch. Oral Biol. 2013, 58, 1751–1760. [Google Scholar] [CrossRef]
- Vernon, H.; Sun, K.; Zhang, Y.; Yu, X.-M.; Sessle, B.J. Central sensitization induced in trigeminal and upper cervical dorsal horn neurons by noxious stimulation of deep cervical paraspinal tissues in rats with minimal surgical trauma. J. Manip. Physiol. Ther. 2009, 32, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Bittmann, F.N.; Dech, S.; Aehle, M.; Schaefer, L.V. Manual Muscle Testing-Force Profiles and Their Reproducibility. Diagnostics 2020, 10, 996. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Ge, M.; Gao, M. Efficacy of low-level laser therapy in the treatment of TMDs: A meta-analysis of 14 randomised controlled trials. J. Oral Rehabil. 2015, 42, 291–299. [Google Scholar] [CrossRef]
- Magri, L.V.; Carvalho, V.A.; Rodrigues, F.C.; Bataglion, C.; Leit Panissi, C.R. Effectiveness of low-level laser therapy on pain intensity, pressure pain threshold, and SF-MPQ indexes of women with myofascial pain. Lasers Med. Sci. 2017, 32, 419–428. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Katz, N.P.; Stucki, G.; Allen, R.R.; Bellamy, N. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005, 113, 9–19. [Google Scholar] [CrossRef]
- Mazzei, L.G.; Bergamaschi, C.C.; Silva, M.T.; Barberato Filho, S.; Fulone, I.; Moura, M.D.G.; Guimaraes, C.; Lopes, L.C. Use of IMMPACT domains in clinical trials of acupuncture for chronic pain: A methodological survey. PLoS ONE 2020, 15, e0231444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lima, C.O.; Caetano, P.L.; Miranda, J.S.; Malta, N.V.; Leite, I.C.G.; Leite, F.P.P. Evaluation of the life quality in patients with Temporomandibular Disorders. Braz. Dent. Sci. 2015, 18, 77–83. [Google Scholar] [CrossRef]
- Bitiniene, D.; Zamaliauskiene, R.; Kubilius, R.; Leketas, M.; Gailius, T.; Smirnovaite, K. Quality of life in patients with temporomandibular disorders. A systematic review. Stomatologija 2018, 20, 3–9. [Google Scholar] [PubMed]
- Chang, H.; Israel, H. Analysis of inflammatory mediators in temporomandibular joint synovial fluid lavage samples of symptomatic patients and asymptomatic controls. J. Oral Maxillofac. Surg. 2005, 63, 761–765. [Google Scholar] [CrossRef]
- Yamaza, T.; Masuda, K.F.; Atsuta, I.; Nishijima, K.; Kido, M.A.; Tanaka, T. Oxidative Stress-induced DNA Damage in the Synovial Cells of the Temporomandibular Joint in the Rat. J. Dent. Res. 2004, 83, 619–662. [Google Scholar] [CrossRef]
- IMMPACT. Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials. Available online: http://www.immpact.org/ (accessed on 30 May 2021).
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Sliney, D.H. Dosimetric concepts for optical radiation. In Dosimetry of Laser Radiation in Medicine and Biology; Proceedings; SPIE: Bellingham, WA, USA, 1989; Volume 10305. [Google Scholar] [CrossRef]
- Jacques, S.L.; Alter, C.A.; Prahl, S.A. Angular dependence of HeNe laser light scattering by human dermis. Lasers Life Sci. 1987, 1, 309–333. [Google Scholar]
- Bachem, A.; Reed, C.I. The penetration of light through human skin. Am. J. Physiol. 1930, 97, 86–91. [Google Scholar] [CrossRef]
- Alvarenga, L.H.; Ribeiro, M.S.; Kato, I.T.; Núñez, S.C.; Prates, R.A. Evaluation of red light scattering in gingival tissue–in vivo study. Photodiagn. Photodyn. Ther. 2018, 23, 32–34. [Google Scholar] [CrossRef]
- Benedicenti, A.; Benedicenti, S. Atlas of Laser Therapy: State of the Art, 4th ed.; Teamwork Media Srl: Villa Carcina, Italy, 2016; pp. 141–152. ISBN 88-89626-02-X. [Google Scholar]
- Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Optical Properties of skin, subcutaneous, and muscle tissues: A review. J. Innov. Opt. Heal. Sci. 2011, 4, 9–38. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Hashmi, J.T.; Huang, Y.Y.; Sharma, S.K.; Kurup, D.B.; De Taboada, L.; Carroll, J.D.; Hamblin, M.R. Effect of pulsing in low-level light therapy. Lasers Surg. Med. 2010, 42, 450–466. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, P.A.; Carroll, J.D. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed. Laser Surg. 2011, 29, 785–787. [Google Scholar] [CrossRef]
- Blasini, M.; Movsas, S.; Colloca, L. Placebo hypoalgesic effects in pain: Potential applications in dental and orofacial pain management. Semin. Orthod. 2018, 24, 259–268. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Zhang, W.; Yi, X.; Liang, C.; Li, X. Efficacy evaluation of low-level laser therapy on temporo-mandibular disorder. West China J. Stomatol. 2011, 29, 393–395. [Google Scholar]
- Svanberg, S. Tissue diagnostics using lasers. In Lasers in Medicine; Waynant, R.W., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 135–169. [Google Scholar]
- Eells, J.T.; Wong-Riley, M.T.; VerHoeve, J.; Henry, M.; Buchman, E.V.; Kane, M.P.; Gould, L.J.; Das, R.; Jett, M.; Hodgson, B.D.; et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 2004, 4, 559–567. [Google Scholar] [CrossRef]
- Pöntinen, P.J. Laser acupuncture. In Lasers in Medicine and Dentistry: Basic and Up-to-Date Clinical Application of Low-Energy-Level Laser Therapy (LLLT); Simunovic, Z., Ed.; Vitgraf: Rijeka, Croatia, 2000; pp. 55–475. [Google Scholar]
- Paolillo, F.R.; Lins, E.C.; Corazza, A.V.; Kurachi, C.; Bagnato, V.S. Thermography applied during exercises with or without infrared light-emitting diode irradiation: Individual and comparative analysis. Photomed. Laser Surg. 2013, 31, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Olivo, S.A.; Fuentes, J.; Major, P.W.; Warren, S.; Thie, N.M.; Magee, D.J. The association between neck disability and jaw disability. J. Oral Rehabil. 2010, 37, 670–679. [Google Scholar] [CrossRef]
- Younger, J.W.; Shen, Y.F.; Goddard, G.; Mackey, S.C. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain 2010, 149, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Jordal, J.M.; Couppé, C.; Chow, R.T.; Tunér, J.; Ljunggren, E.A. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust. J. Physiother. 2003, 49, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Doerrer, A.; Figart, F. TMJ syndrome: Is it compensable? Rehabil. Nurs. 1991, 16, 23–26. [Google Scholar] [CrossRef] [PubMed]
- CDC—Concept—Health-Related Quality of Life. Available online: https://www.cdc.gov/hrqol/concept.htm (accessed on 14 May 2021).
- Chang, W.D.; Lee, C.L.; Lin, H.Y.; Hsu, Y.C.; Wang, C.J.; Lai, P.T. A meta-analysis of clinical effects of low-level laser therapy on temporomandibular joint pain. J. Phys. Ther. Sci. 2014, 26, 1297–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.Z.; Jia, J.; Jin, L.; Li, J.H.; Wang, Z.Y.; Cao, D.Y. Low-level laser therapy for temporomandibular disorders: A systematic review with meta-analysis. Pain Res. Manag. 2018, 2018, 4230583. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Assessment of Outcome Measures | Primary Outcomes | Secondary Outcomes | |
|---|---|---|---|
| Pain Reduction | Functional Improvement | Anxiety/Depression and QoL | |
| Qualitative (patient-reported outcomes; subjective) | Visual analogue scale (VAS) Numerical scale of pain OHIP/TMD questionnaire McGill pain questionnaire Symptom severity index Orofacial myofunctional evaluation protocol with scores (OMES) | Patient-specific functional scale | Euro Qol-5D 5L Beck anxiety inventory (BAI) Pain distress scale |
| Quantitative (objective) | Kaplan–Meier method Pressure pain threshold (PPT) (dial algometer) Power algometer | Kaplan–Meier method Jaw kinesiology Craniomandibular index (CMI) Colorimetric capsules Electromyography (EMG) Digital pachymeter (Digimess) to measure the vertical and horizontal movements Stethoscope (crepitation) Helkimo index Anamnestic questionnaire Computerized photogrammetry Masticatory test Active range of motion (AROM) index Digital calliper Flexible millimetre ruler Millimetre ruler RDC/TDM | |
| TMJ Synovial fluid analysis (immunological profile) | To evaluate the levels of the following data: oxidative stress, IL-1,6,8 (Interleukin-1,6,8), TNF-α (Tumor necrosis factor- alpha) and β, MM-1,2,9 (Mandibular movement-1,2,9), VEGF (Vascular endothelial growth factor), TGF-β1 (Transforming growth factor-β1), IGF-I (Insulin-like growth factor-I) | ||
| Essential Reported Parameters | Desirable Reported Parameters | ||
|---|---|---|---|
| Device Information | Irradiation Parameters | Treatment Parameters | Energy per Pulse (J) |
| Manufacturer | Wavelength (nm) | Beam spot size at target (cm2) | Polarisation |
| Model identifier | Spectral bandwidth (nm) | Irradiance at target (mW/cm2) | Aperture diameter (cm) |
| Emitters type (e.g., nGaAlP LED, GaAlAs LASER, KTP LASER) | Operating mode (CW, pulsed, super pulsed) | Exposure duration (sec) | Irradiance at aperture (mW/cm2) |
| Number of emitters | Frequency (Hz) | Radiant exposure (J/cm2) | Beam divergence (°) |
| Spatial distribution of emitters. (e.g., 4 emitters spaced 2 cm apart in a square pattern). | Pulse width (second) | Radiant energy (J) | Beam shape |
| Beam delivery system (e.g., fibreoptic, free air/scanned, hand-held probe). | Duty cycle (%) | Number of points irradiated | Scanning technique |
| Beam profile | Area irradiated (cm2) | Speed of movement | |
| Application technique | |||
| Number and frequency of treatment sessions | |||
| Total radiant energy (J) | |||
| Delivery Route of PBM Irradiation ** | Affected Regions ** | Treatment Area and No. of TP (Optimal Target Tissue), Depending on Palpable Areas, Including Cervical Muscles ** (Figure 13) | PBM Device Characteristics, Application and Treatment Protocol ** | Frequency and Treatment Duration Protocol ** |
|---|---|---|---|---|
| Extraoral (EO) | TMJ | External acoustic meatus: 1 Periauricular: 1 | Laser λ: 790–830 nm [65,73] Therapeutic power output (at the target): 100–500 mW; emission mode: CW; irradiated area: 0.5–1 cm2 Contact on skin surface Exposure time: 30–60 s A firm pressure on skin surface applied to increase light penetration depth Laser and LEDs: [75] Red (630 ± 10 nm) (9 J/point, 18 J/cm2, 150 mW, 300 mW/cm2/point, 0.5 cm2), IR (850 ± 10 nm) LEDs, (9 J/point, 18 J/cm2, 150 mW, 300 mW/cm2/point, 0.5 cm2) IR laser (780 nm); positive control (4.2 J/point, 70 mW, 0.04 cm2, 1.7 W/cm2/point, 105 J/cm2), Fluence: 18 and 105 J/cm2 | 2–3 times a week At least 4 consecutive weeks (total of 8–10 sessions), depending on status of presented symptoms (acute or chronic) |
| TMJ-associated regions | Superior, anterior, lateral, posterior, postero-inferior to the condyle: 5 | |||
| Masticatory muscles | Temporalis: 3 Masseter muscle: 5 LPM: 2, MPM: 2 | |||
| Cervical muscles | Trapezius: 4 Sternocleidomastoid muscle: 5 Anterior belly of digastric muscle: 2 Posterior belly of digastric muscle: 3 | |||
| Intraoral (IO) | Masticatory muscles | Superficial head of the MPM: 1 LPM: 1 Insertion of temporalis muscle: 2 | Laser (905 nm) and LEDs (4 clusters of 640 nm (Red) + 4 clusters of 875 nm (IR)) [95] 905 nm diode laser: 0.9 mW (MOPO), 1000 Hz, 0.4 cm2, 300 s, super-pulsed 640 nm LED: 15 mW (MOPO), 2 Hz, 0.9 cm2, 300 s, pulsed 875 nm LED: 17.5 mW (MOPO), 16 Hz, 0.9 cm2, 300 s, pulsed Adapter with aperture of 0.394 cm2 Dose: 99.67 J/cm2/point | |
| Combined IO and EO | Superficial head of the MPM: 1 (IO) LPM:1 (IO) Insertion of temporalis muscle: 2 (IO) Masseter muscle: 5 (EO) | Laser: combined λ 660 + λ 890 nm [67] 660 nm (CW, 6–10 J/point, 17.3 mW, 360 s exposure time), 890 nm (pulsed, 1500 Hz, 9.8 W, 1 J/cm2, 600 s), mild tissue pressure applied, irradiated area 0.6 cm, dose: 6–10 J/cm2 (deep-seated tissue) and 1–4 J/cm2 (superficial-seated tissue) | ||
| Key Factors ** | Suggested Recommendation ** | Description and Citation of Scientifc Evidence ** (Manuscript) | |
|---|---|---|---|
| Population characteristics | Age range | Paediatric cohort: <18 years old Adult cohort: 18–40 years old 41–60 years old >60 years old | Refer to Section 4.1 |
| Gender | Either female or only male category for each study. Mixed-gender subjects, depending on aims and objectives of the study | ||
| Racial background | It is noteworthy that skin colour plays an important role in the scattering and absorption of the photonic energy. It can have a great impact on PBM dosimetry | ||
| Optical properties of the target tissue | Identify the consistency, structure, thickness, skin colour and absorption/scattering coefficient of the target tissue | Refer to Section 4.3 | |
| Sample size |
| Refer to Section 4.1 and Section 4.2.1 | |
| Randomisation and blinding processes | Two independent blind investigators to assess the variables at all timepoints (double-blind) and record the data. Robust randomised process. Parallel arm study design. | Refer to Section 4.2.1 | |
| Comparable arms of the study | Placebo/sham PBM, as a comparable arm in TMD study design, is essential to validate the optimal outcome. It assists in providing standarised and reproducible data | [137] | |
| Presented symptoms | Pain:
Functional disability:
Anxiety/depression |
| Refer to Section 4.2.1 and Table S2 |
| Diagnostic criteria | Combining 2 tools: RDC/TMD and diagnostic manual muscle testing (MMT) for cervical muscles assessment | Refer to Section 4.2.2 | |
| Standardised laser protocol | Based on gathered evidence-based practice and science, 11 out of 44 studies utilised power meter and placebo/sham PBM; recommendations of PBMT protocols suggested for further research | Refer to Table 4 and Section 4.5 | |
| Light device standardisation | Standardised prototype development for each involved light source in the study | ||
| Route of delivery of PBM irradiation | Identifying the consistency, structure, thickness, skin colour and absorption/scattering coefficient of the target tissues (optical properties) of each of the following PBM delivery rout(s), prior to setting up the PBM parameter protocols: Transmucosal approach (IO) Transcutaneous approach (EO) Combination of the above approaches | Refer to Section 4.4 and Table 4 | |
| Unilateral/bilateral PBM irradiation | Regardless of unilateral or bilateral TMD symptoms, PBM irradiation needs to be applied bilaterally, due to the concept of compensation effects | [147] | |
| Trigger Points (TP) | Affected sites Number of the TP/site | All the TP need to be addressed as follows: EO and IO muscles’ contribution to TMD, including the masticatory muscles, as well as the cervical (palpable or unpalpable). The number of the TP depends on the origin and insertion of each muscle, its location and volume | Refer to Section 4.5.4 and Figure 13 |
| The investigated variables and follow-up timepoints |
| Refer to Section 4 | |
| Outcome measures |
| Refer to Table S2 and these citations: [124,125,126] | |
| Reported data | Documentation of essential and desirable PBM parameters is pivotal for reproducibility and standardisation | Refer to Table 2 [135] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, R.; Dalvi, S.; Bensadoun, R.J.; Benedicenti, S. Role of Photobiomodulation Therapy in Modulating Oxidative Stress in Temporomandibular Disorders. A Systematic Review and Meta-Analysis of Human Randomised Controlled Trials. Antioxidants 2021, 10, 1028. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10071028
Hanna R, Dalvi S, Bensadoun RJ, Benedicenti S. Role of Photobiomodulation Therapy in Modulating Oxidative Stress in Temporomandibular Disorders. A Systematic Review and Meta-Analysis of Human Randomised Controlled Trials. Antioxidants. 2021; 10(7):1028. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10071028
Chicago/Turabian StyleHanna, Reem, Snehal Dalvi, René Jean Bensadoun, and Stefano Benedicenti. 2021. "Role of Photobiomodulation Therapy in Modulating Oxidative Stress in Temporomandibular Disorders. A Systematic Review and Meta-Analysis of Human Randomised Controlled Trials" Antioxidants 10, no. 7: 1028. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10071028