The Fine-Tuned Release of Antioxidant from Superparamagnetic Nanocarriers under the Combination of Stationary and Alternating Magnetic Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
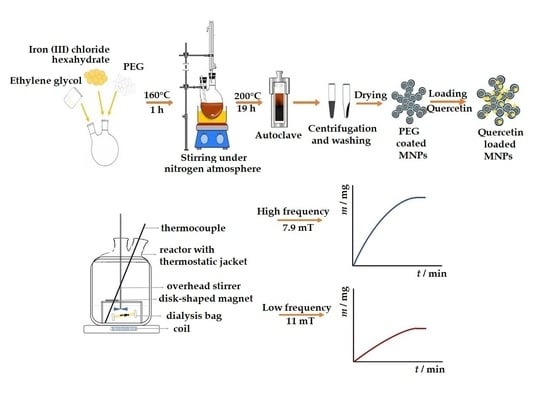
2.2. Synthesis of Bare and PEG Coated Magnetite MNPs
2.3. Characterization of Synthesized Magnetite MNPs
2.4. The Determination of Loaded Quercetin into Magnetic Mesoporous MNPs
Size Distribution of Magnetic MNPs Using Dynamic and Electrophoretic Light Scattering
2.5. Release Study of Quercetin under Stationary and Alternating Magnetic Fields
3. Results and Discussion
3.1. Characterization of Synthesized Magnetite MNPs
3.2. Loading of Flavonoid into Magnetite MNPs
3.3. Homogeneity and Stability of Synthesized Mesoporous MNPs
3.4. Release Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Dziubinska, H.; Król, E.; Trebacz, K.; Jarosz-Wilkolazka, A.; Paduch, R.; Gawron, A.; Gruszecki, W. Characteristics of quercetin interactions with liposomal and vacuolar membranes. Biochim. Biophys. Acta 2014, 1838, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Barreto, A.C.H.; Santiago, V.R.; Mazzetto, S.E.; DeNardin, J.C.; Lavín, R.; Mele, G.; Ribeiro, M.E.N.P.; Vieira, I.G.P.; Gonçalves, T.; Ricardo, N.M.P.S.; et al. Magnetic nanoparticles for a new drug delivery system to control quercetin releasing for cancer chemotherapy. J. Nanoparticle Res. 2011, 13, 6545–6553. [Google Scholar] [CrossRef]
- Mandić, L.; Sadžak, A.; Strasser, V.; Baranović, G.; Jurašin, D.D.; Sikirić, M.D.; Šegota, S. Enhanced Protection of Biological Membranes during Lipid Peroxidation: Study of the Interactions between Flavonoid Loaded Mesoporous Silica Nanoparticles and Model Cell Membranes. Int. J. Mol. Sci. 2019, 20, 2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Berlier, G.; Gastaldi, L.; Ugazio, E.; Miletto, I.; Iliade, P.; Sapino, S. Stabilization of quercetin flavonoid in MCM-41 mesoporous silica: Positive effect of surface functionalization. J. Colloid Interface Sci. 2013, 393, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Elmasry, S.A.; Elgawish, M.A.; El-Shawy, O.E.; Askar, M.A.; Helmy, E.A.; Rashed, L.A. Biologically Synthesized Quercetin Loaded Magnetite Nanoparticles Enhanced Cytotoxicity and Radiosensitivity of Cancer Cells in Vitro. J. Chem. Pharm. Res. 2016, 8, 758–771. [Google Scholar]
- Hu, J.; Obayemi, J.; Malatesta, K.; Kosmrlj, A.; Soboyejo, W. Enhanced cellular uptake of LHRH-conjugated PEG-coated magnetite nanoparticles for specific targeting of triple negative breast cancer cells. Mater. Sci. Eng. C 2018, 88, 32–45. [Google Scholar] [CrossRef]
- Krukemeyer, M.G.; Krenn, V.; Huebner, F.; Wagner, W.; Resch, R. History and Possible Uses of Nanomedicine Based on Nanoparticles and Nanotechnological Progress. J. Nanomed. Nanotechnol. 2015, 6, 336. [Google Scholar] [CrossRef] [Green Version]
- Stevanović, M.; Lukić, M.J.; Stanković, A.; Filipović, N.; Kuzmanović, M.; Janićijević, Ž. Biomedical inorganic nanoparticles: Preparation, properties, and perspectives. Biomed. Mater. Eng. 2019, 1–46. [Google Scholar] [CrossRef]
- Favela-Camacho, S.E.; Samaniego, J.E.; Godínez-García, A.; Avilés-Arellano, L.M.; Pérez-Robles, J.F. How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 29–35. [Google Scholar] [CrossRef]
- Unsoy, G.; Gunduz, U.; Oprea, O.; Ficai, D.; Sonmez, M.; Radulescu, M.; Alexie, M.; Ficai, A. Magnetite: From Synthesis to Applications. Curr. Top. Med. Chem. 2015, 15, 1622–1640. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Lim, H.N.; Gowthaman, N.; Rahman, M.B.A.; Abdullah, C.A.C.; Muthoosamy, K. In-situ surface functionalization of superparamagnetic reduced graphene oxide—Fe3O4 nanocomposite via Ganoderma lucidum extract for targeted cancer therapy application. Appl. Surf. Sci. 2020, 512, 145738. [Google Scholar] [CrossRef]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef]
- Jesus, P.D.C.C.D.; Pellosi, D.; Tedesco, A. Magnetic nanoparticles: Applications in biomedical processes as synergic drug-delivery systems. Mater. Biomed. Eng. 2019, 371–396. [Google Scholar] [CrossRef]
- Widder, K.J.; Morris, R.M.; Poore, G.A.; Howard, D.P.; Senyei, A.E. Selective targeting of magnetic albumin microspheres containing low-dose doxorubicin: Total remission in Yoshida sarcoma-bearing rats. Eur. J. Cancer Clin. Oncol. 1983, 19, 135–139. [Google Scholar] [CrossRef]
- Mohanta, S.C.; Saha, A.; Devi, P.S. PEGylated Iron Oxide Nanoparticles for pH Responsive Drug Delivery Application. Mater. Today Proc. 2018, 5, 9715–9725. [Google Scholar] [CrossRef]
- García-Jimeno, S.; Estelrich, J. Ferrofluid based on polyethylene glycol-coated iron oxide nanoparticles: Characterization and properties. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 420, 74–81. [Google Scholar] [CrossRef]
- Revia, R.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Anarjan, N.; Vaghari, H.; Sayyar, Z.; Berenjian, A. A biotechnological perspective on the application of iron oxide nanoparticles. Nano Res. 2016, 9, 2203–2225. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Schroeder, G. Preparation of multifunctional cascade iron oxide nanoparticles for drug delivery. Mater. Chem. Phys. 2018, 211, 34–41. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic Nanoparticle-based drug delivery for cancer therapy, Magnetic Nanoparticle-based Drug Delivery for Cancer Therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Tansık, G.; Yakar, A.; Gündüz, U. Tailoring magnetic PLGA nanoparticles suitable for doxorubicin delivery. J. Nanoparticle Res. 2013, 16, 2171. [Google Scholar] [CrossRef]
- Podaru, G.; Chikan, V. Magnetism in Nanomaterials: Heat and Force from Colloidal Magnetic Particles. In Magnetic Nanomaterials: Applications in Catalysis and Life Sciences; Bossmann, H.S., Wang, H., Eds.; The Royal Society of Chemistry: Croydon, UK, 2017; pp. 1–24. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V.; Vázquez, A.; Peña, Y.; Gómez, I. Solubilization, dispersion and stabilization of magnetic nanoparticles in water and non-aqueous solvents: Recent trends. RSC Adv. 2014, 4, 45354–45381. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Hildgen, P.; Banquy, X. Assessment of PEG on polymeric particles surface, a key step in drug carrier translation. J. Control. Release 2014, 185, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Yang, Q.; Dong, Z.; Zhang, J.; Zhang, W.; Wang, Q.; Tan, S.; Smyth, H.D.C. Magnetically triggered drug release from nanoparticles and its applications in anti-tumor treatment. Drug Deliv. 2017, 24, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Luo, B.; Xu, S.; Luo, A.; Wang, W.-R.; Wang, S.-L.; Guo, J.; Lin, Y.; Zhao, D.; Wang, C.-C. Mesoporous Biocompatible and Acid-Degradable Magnetic Colloidal Nanocrystal Clusters with Sustainable Stability and High Hydrophobic Drug Loading Capacity. ACS Nano 2011, 5, 1428–1435. [Google Scholar] [CrossRef]
- Luo, B.; Xu, S.; Ma, W.-F.; Wang, W.-R.; Wang, S.-L.; Guo, J.; Yang, W.-L.; Hu, J.-H.; Wang, C.-C. Fabrication of magnetite hollow porous nanocrystal shells as a drug carrier for paclitaxel. J. Mater. Chem. 2010, 20, 7107–7113. [Google Scholar] [CrossRef]
- Šegota, S.; Baranović, G.; Mustapić, M.; Strasser, V.; Domazet Jurašin, D.; Crnolatac, I.; Al Hossain, M.S.; Dutour Sikirić, M. The role of spin-phonon coupling in enhanced desorption kinetics of antioxidant flavonols from magnetic nanoparticles aggregates. J. Magn. Magn. Mater. 2019, 490, 165530. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertz, D.; Sandre, O.; Bégin-Colin, S. Drug releasing nanoplatforms activated by alternating magnetic fields. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1617–1641. [Google Scholar] [CrossRef] [PubMed]
- Razmara, R.S.; Daneshfar, A.; Sahraei, R. Solubility of Quercetin in Water + Methanol and Water + Ethanol from (292.8 to 333.8) K. J. Chem. Eng. Data 2010, 55, 3934–3936. [Google Scholar] [CrossRef]
- Widdrat, M.; Kumari, M.; Tompa, Éva; Pósfai, M.; Hirt, A.; Faivre, D. Keeping Nanoparticles Fully Functional: Long-Term Storage and Alteration of Magnetite. ChemPlusChem 2014, 79, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Gronczewska, E.; Defort, A.; Kozioł, J. Kinetics of Ibuprofen Release from Magnetic Nanoparticles Coated with Chitosan, Peg and Dextran. Pharm. Chem. J. 2016, 50, 491–499. [Google Scholar] [CrossRef]
- Hariani, P.L.; Faizal, M.; Ridwan, R.; Marsi, M.; Setiabudidaya, D. Synthesis and Properties of Fe3O4 Nanoparticles by Co-precipitation Method to Removal Procion Dye. Int. J. Environ. Sci. Dev. 2013, 4, 336–340. [Google Scholar] [CrossRef]
- Yang, J.; Zou, P.; Yang, L.; Cao, J.; Sun, Y.; Han, D.; Yang, S.; Wang, Z.; Chen, G.; Wang, B.; et al. A comprehensive study on the synthesis and paramagnetic properties of PEG-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2014, 303, 425–432. [Google Scholar] [CrossRef]
- Masoudi, A.; Hosseini, H.R.M.; Shokrgozar, M.A.; Ahmadi, R.; Oghabian, M.A. The effect of poly(ethylene glycol) coating on colloidal stability of superparamagnetic iron oxide nanoparticles as potential MRI contrast agent. Int. J. Pharm. 2012, 433, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [Green Version]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathishkumar, P.; Li, Z.; Govindan, R.; Jayakumar, R.; Wang, C.; Gu, F.L. Zinc oxide-quercetin nanocomposite as a smart nano-drug delivery system: Molecular-level interaction studies. Appl. Surf. Sci. 2021, 536, 147741. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, M.; Ma, R.; Cheng, H.; Yang, S.; Li, Y.Y.; Zapien, J.A. Evaporation-induced synthesis of carbon-supported Fe3O4 nanocomposites as anode material for lithium-ion batteries. CrystEngComm 2013, 15, 1324–1331. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, T.S.; Son, K.S.; Maeng, W.-J.; Kim, H.-S.; Ryu, M.; Lee, S.Y. The effect of UV-assisted cleaning on the performance and stability of amorphous oxide semiconductor thin-film transistors under illumination. Appl. Phys. Lett. 2011, 98, 12107. [Google Scholar] [CrossRef]
- Swain, A.K.; Pradhan, L.; Bahadur, D. Polymer Stabilized Fe3O4-Graphene as an Amphiphilic Drug Carrier for Thermo-Chemotherapy of Cancer. ACS Appl. Mater. Interfaces 2015, 7, 8013–8022. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avedian, N.; Zaaeri, F.; Daryasari, M.P.; Javar, H.A.; Khoobi, M. pH-sensitive biocompatible mesoporous magnetic nanoparticles labeled with folic acid as an efficient carrier for controlled anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2018, 44, 323–332. [Google Scholar] [CrossRef]
- Selvaraj, S.; Shanmugasundaram, S.; Maruthamuthu, M.; Venkidasamy, B.; Shanmugasundaram, S. Facile Synthesis and Characterization of Quercetin-Loaded Alginate Nanoparticles for Enhanced In Vitro Anticancer Effect Against Human Leukemic Cancer U937 Cells. J. Clust. Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.; Layek, B.; Nirzhor, S.S.R.; Prabha, S. Nanoparticle-Mediated Photodynamic Therapy for Mixed Biofilms. J. Nanomater. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Engen, A.; Maeda, J.; Wozniak, D.E.; Brents, C.A.; Bell, J.J.; Uesaka, M.; Aizawa, Y.; Kato, T.A. Induction of cytotoxic and genotoxic responses by natural and novel quercetin glycosides. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 784–785, 15–22. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12802–12811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolová, R.; Degano, I.; Ramešová, Š.; Bulíčková, J.; Hromadová, M.; Gál, M.; Fiedler, J.; Valášek, M. The oxidation mechanism of the antioxidant quercetin in nonaqueous media. Electrochim. Acta 2011, 56, 7421–7427. [Google Scholar] [CrossRef]
- Schwarzl, R.; Du, F.; Haag, R.; Netz, R.R. General method for the quantification of drug loading and release kinetics of nanocarriers. Eur. J. Pharm. Biopharm. 2017, 116, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting drug release kinetics from nanocarriers inside dialysis bags. J. Control. Release 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]









| Bare MNPs | PEG Coated MNPs | Quercetin Loaded PEG_MNPs | |
|---|---|---|---|
| ζ/mV | −30.6 ± 0.7 | −35.1 ± 1.5 | −31.3 ± 0.8 |
| dh/nm | 877 ± 105 | 762 ± 92 | 681 ± 73 |
| PDI | 0.54 ± 0.10 | 0.47 ± 0.10 | 0.52 ± 0.07 |
| B/mT | falt.mag.f./kHz | θ/°C | k/min−1 | m0/mg |
|---|---|---|---|---|
| 11.0 | 100 | 25 | 0.0029 ± 0.0004 | 0.82 ± 0.01 |
| 30 | 0.0034 ± 0.0013 | 1.38 ± 0.30 | ||
| 37 | 0.0038 ± 0.0013 | 1.88 ± 0.25 | ||
| 50 | 25 | 0.0022 ± 0.0004 | 1.03 ± 0.30 | |
| 30 | 0.0019 ± 0.0001 | 1.41 ± 0.01 | ||
| 37 | 0.0025 ± 0.0015 | 3.3 ± 1.4 | ||
| 10 | 25 | 0.0024 ± 0.0012 | 1.07 ± 0.34 | |
| 30 | 0.0032 ± 0.0009 | 0.97 ± 0.20 | ||
| 37 | 0.0033 ± 0.0003 | 1.73 ± 0.22 | ||
| 7.9 | 100 | 25 | 0.0015 ± 0.0002 | 1.79 ± 0.14 |
| 30 | 0.0032 ± 0.0003 | 1.15 ± 0.44 | ||
| 37 | 0.0052 ± 0.0011 | 1.50 ± 0.39 | ||
| 50 | 25 | 0.0022 ± 0.0007 | 1.20 ± 0.26 | |
| 30 | 0.0022 ± 0.0008 | 1.86 ± 0.35 | ||
| 37 | 0.0025 ± 0.0002 | 1.21 ± 0.01 | ||
| 10 | 25 | 0.0043 ± 0.0003 | 1.13 ± 0.08 | |
| 30 | 0.0021 ± 0.0006 | 1.30 ± 0.24 | ||
| 37 | 0.0016 ± 0.0005 | 1.64 ± 0.52 | ||
| 0 | 0 | 25 | 0.0022 ± 0.0004 | 1.03 ± 0.30 |
| 30 | 0.0019 ± 0.0001 | 1.41 ± 0.01 | ||
| 37 | 0.0025 ± 0.0015 | 2.25 ± 1.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandić, L.; Sadžak, A.; Erceg, I.; Baranović, G.; Šegota, S. The Fine-Tuned Release of Antioxidant from Superparamagnetic Nanocarriers under the Combination of Stationary and Alternating Magnetic Fields. Antioxidants 2021, 10, 1212. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10081212
Mandić L, Sadžak A, Erceg I, Baranović G, Šegota S. The Fine-Tuned Release of Antioxidant from Superparamagnetic Nanocarriers under the Combination of Stationary and Alternating Magnetic Fields. Antioxidants. 2021; 10(8):1212. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10081212
Chicago/Turabian StyleMandić, Lucija, Anja Sadžak, Ina Erceg, Goran Baranović, and Suzana Šegota. 2021. "The Fine-Tuned Release of Antioxidant from Superparamagnetic Nanocarriers under the Combination of Stationary and Alternating Magnetic Fields" Antioxidants 10, no. 8: 1212. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10081212