Medicinal Plants Galega officinalis L. and Yacon Leaves as Potential Sources of Antidiabetic Drugs
Abstract
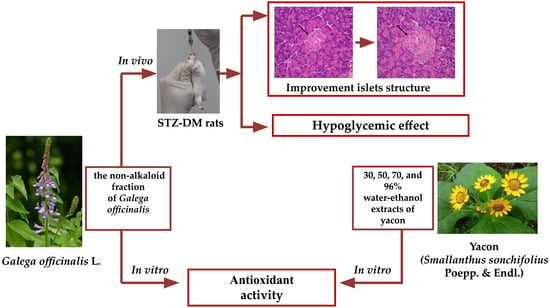
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Galega officinalis Extract and Its Stabilization by Adding the PS (Pseudomonas sp.) Biocomplex
2.3. Induction of Diabetes
2.4. Experimental Animals
2.5. Determination of Blood Glucose Concentration
2.6. Immuno-Enzymatic Analysis of Insulin, C-Peptide, and Tumor Necrosis Factor Alpha Contents
2.7. Glucose Tolerance Test Assay
2.8. Determination of Glycosylated Hemoglobin Content
2.9. Light Microscopy of Cells of the Pancreas
2.10. Preparation of Yacon Extracts
2.11. Total Phenolic Compound Content (TPC) Analysis
2.12. DPPH Assay
2.13. ABTS Assay
2.14. FRAP Assay
2.15. Statistical Analysis of Results
3. Results
3.1. Hypoglycemic Effect of Galega officinalis Extract
3.2. In Vitro Antioxidant Effect of Non-Alkaloid Fraction of Galega Officinalis and Yacon Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Zatalia, S.R.; Sanusi, H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med. Indones. 2013, 45, 141–147. [Google Scholar]
- Liu, Q.; Wang, S.; Cai, L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J. Diabetes Investig. 2014, 5, 623–634. [Google Scholar] [CrossRef]
- Lheureux, P.; Even-Adin, D.; Askenasi, R. Current Status of Antidotal Therapies in Acute Human Intoxications. Acta Clin. Belg. 1990, 45, 29–47. [Google Scholar] [CrossRef]
- Eddouks, M.; Bidi, A.; El Bouhali, B.; Hajji, L.; Zeggwagh, N.A. Antidiabetic plants improving insulin sensitivity. J. Pharm. Pharm. 2014, 66, 1197–1214. [Google Scholar] [CrossRef]
- Patel, D.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Preetha, P.P.; Devi, V.G.; Rajamohan, T. Hypoglycemic and antioxidant potential of coconut water in experimental diabetes. Food Funct. 2012, 3, 753–757. [Google Scholar] [CrossRef]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef]
- Gresham, A.; Booth, K. Poisoning of sheep by goat’s rue. Vet. Rec. 1991, 129, 197–198. [Google Scholar] [CrossRef]
- Neef, H.; Declercq, P.; Laekeman, G. Hypoglycaemic activity of selected European plants. Phytother. Res. 1995, 9, 45–48. [Google Scholar] [CrossRef]
- Rasekh, H.R.; Nazari, P.; Kamli-Nejad, M.; Hosseinzadeh, L. Acute and subchronic oral toxicity of Galega officinalis in rats. J. Ethnopharmacol. 2008, 116, 21–26. [Google Scholar] [CrossRef]
- Lapinina, L.A.; Sysoeva, T.F. Sugar-lowering activity of Galega officinalis. Pharm. J. 1961, 5, 52–56. (In Ukrainian) [Google Scholar]
- Lapinina, N.A. Isolation and Study of Physiologically Active Compounds of Galega officinalis as a Raw Material for Obtaining a Hypoglycemic Drug. Ph.D. Thesis, The Kharkiv University of Pharmacy, Kharkiv, Ukraine, 1972. (In Russian). [Google Scholar]
- Barcellona, C.S.; Cabrera, W.M.; Honoré, S.M.; Mercado, M.I.; Sánchez, S.S.; Genta, S.B. Safety assessment of aqueous extract from leaf Smallanthus sonchifolius and its main active lactone, enhydrin. J. Ethnopharmacol. 2012, 144, 362–370. [Google Scholar] [CrossRef]
- Mishchenko, L.T. New vegetable and medicinal culture in Ukraine. Sci. Bull. Natl. Univ. Life Environ. Sci. Ukr. 2012, 180, 250–256. (In Ukrainian) [Google Scholar]
- Marchyshyn, S.; Hudz, N.; Dakhym, I.; Husak, L.; Mishchenko, L. Analysis of phenolic compounds from Polymnia sonchifolia Poepp. et Endl. leaves by HPLC-method. Pharma Innov. J. 2017, 6, 980–983. [Google Scholar]
- Sybirna, N.; Vildanova, R.; Shulga, O.; Sheglova, N.; Karpenko, O.; Khokhla, M.; Hachkova, H.; Lupak, M. A Method for Preparing of Phytopreparation Based on Non-Alkaloid Fraction of Galega officinalis Extract. U.S. Patent No. UA 101202, 25 August 2015. [Google Scholar]
- Yeh, S.T. Using Trapezoidal Rule for the Area under a Curve Calculation. Proc. Twenty Seventh Annu. Sas. 2002, 27, 229–237. [Google Scholar]
- van Kampen, E.J.; Zijlstra, W.G. Spectrophotometry of Hemoglobin and Hemoglobin Derivatives. Adv. Clin. Chem. 1983, 23, 199–257. [Google Scholar]
- Korzhevsky, D.E.; Gilyarov, A.V. Fundamentals Principles of Histological Technique; SpetsLit Publishing House Ltd.: St. Petersburg, Russia, 2010; pp. 8–44. (In Russian) [Google Scholar]
- Sarkisov, D.S.; Perov, Y.L. Microscopic Technique; Medicine: Moskow, Russia, 1996; pp. 375–419. (In Russian) [Google Scholar]
- Merkulov, G.A. Histopathologic Technique Course; Medicine: Moskow, Russia, 1969; pp. 369–405. (In Russian) [Google Scholar]
- Williams, M.A. Quantitative methods in biology. In Practical Methods in Electron Microscopy; Glauert, A.M., Ed.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1977; pp. 48–62. [Google Scholar]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Pellegrini, R.R.; Proteggente, N.A.; Pannala, A.; Yang, M. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, A.S.; McDaniel, M.L. Identifying the links between obesity, insulin resistance and β-cell function: Potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes: Cytokines, insulin resistance and β-cell function. Eur. J. Clin. Investig. 2002, 32, 24–34. [Google Scholar] [CrossRef]
- Mishchenko, L.; Khokhla, M.; Hachkova, H.; Sybirna, N.; Vildanova, R.; Shulga, O. Yacon’s (Smallanthus sonchifolius Poepp. & Endl.) effects on postprandial glucose under experimental diabetes mellitus. Ukr. Biopharm J. 2016, 3, 63–69. [Google Scholar]
- Khokhla, M.; Horbulinska, O.; Hachkova, H.; Mishchenko, L.; Shulga, O.; Vildanova, R.; Sybirna, N. Yacon (Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson) Improved Erythrocyte Resistance to Oxidative Stress in Streptozotocin-induced Diabetic Rats. Adv. Diabetes Metab. 2015, 3, 17–25. [Google Scholar]
- Dent, M.; Dragovic-Uzelac, V.; Penic, M.; Bosiljkov, Y.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Librán, C.M.; Mayor, L.; Garcia-Castello Esperanza, M.; Vidal-Brotons, D. Polyphenol extraction from grape wastes: Solvent and pH effect. Agric. Sci. 2013, 4, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Men’shchikova, E.V.; Lankin, V.Z.; Kandalintseva, N.V. Phenolic Antioxidants in Biology and Medicine. Structure, Properties, Mechanisms of Action; LAP: Saarbrucken, Germany, 2012; pp. 61–117. [Google Scholar]
- von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Aybar, M.J.; Sánchez Riera, A.N.; Grau, A.; Sánchez, S.S. Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. J. Ethnopharmacol. 2001, 74, 125–132. [Google Scholar] [CrossRef]
- Baroni, S.; Suzuki-Kemmelmeier, F.; Caparroz-Assef, S.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Effect of crude extracts of leaves of Smallanthus sonchifolius (yacon) on glycemia in diabetic rats. Rev. Bras. Cienc. Farm. 2008, 44, 521–530. [Google Scholar] [CrossRef]
- Eisenbarth, G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [PubMed]
- Kukreja, A.; Maclaren, N.K. Autoimmunity and diabetes. J. Clin. Endocrinol. Metab. 1999, 84, 4371–4378. [Google Scholar] [CrossRef]
- Mandrup-Poulsen, T.; Bendtzen, K.; Dinarello, C.A.; Nerup, J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J. Immunol. 1987, 139, 4077–4082. [Google Scholar] [PubMed]
- Bruun, C.; Heding, P.E.; Rønn, S.G.; Frobøse, H.; Rhodes, C.J.; Mandrup-Poulsen, T.; Billestrup, N. Suppressor of cytokine signalling-3 inhibits tumor necrosis factor-alpha induced apoptosis and signalling in beta cells. Mol. Cell. Endocrinol. 2009, 311, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Yang, Z.D.; Smith, K.M.; Carter, J.D.; Nadler, J.L. Activation of 12- lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetology 2005, 48, 486–495. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, K.; Han, S.; Shioda, S.; Hirai, M.; Nishizawa, M.; Handa, H. MafA Is a Glucose-regulated and Pancreatic β-Cell-specific Transcriptional Activator for the Insulin Gene. J. Biol. Chem. 2002, 277, 49903–49910. [Google Scholar] [CrossRef] [Green Version]
- Olbrot, M.; Rud, J.; Moss, L.G.; Sharma, A. Identification of -cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc. Natl. Acad. Sci. USA 2002, 99, 6737–6742. [Google Scholar] [CrossRef] [Green Version]
- Lupak, M.I.; Khokhla, M.R.; Hachkova, G.Y.; Kanyuka, O.P.; Klymyshyn, N.I.; Chajka, Y.P.; Skybitska, M.I.; Sybirna, N.O. The alkaloid-free fraction from Galega officinalis extract prevents oxidative stress under experimental diabetes mellitus. Ukr. Biochem. J. 2015, 87, 78–86. (In Ukranian) [Google Scholar] [CrossRef]
- Bonner-Weir, S. Perspective: Postnatal Pancreatic β Cell Growth. Endocrinology 2000, 141, 1926–1929. [Google Scholar] [CrossRef]
- Dickson, L.M.; Rhodes, C.J. Pancreatic β-cell growth and survival in the onset of type 2 diabetes: A role for protein kinase B in the Akt? Am. J. Physiol. Endocrinol. Metab. 2004, 287, E192–E198. [Google Scholar] [CrossRef]
- Lupak, M.; Khokhla, M.; Hachkova, G.; Shulga, O.; Sheglova, N.; Vildanova, R.; Zyn, A.; Sybirna, N. Application of biogenic surfactants for alkaloid-free fraction from Galega officinalis extract stabilization. Stud. Biol. 2015, 9, 5–16. (In Ukranian) [Google Scholar] [CrossRef] [Green Version]
- Elmazar, M.M.; El-Abhar, H.S.; Schaalan, M.F.; Farag, N.A. Phytol/Phytanic Acid and Insulin Resistance: Potential Role of Phytanic Acid Proven by Docking Simulation and Modulation of Biochemical Alterations. PLoS ONE 2013, 8, e45638. [Google Scholar] [CrossRef] [Green Version]
- Sarkodie, J.A.; Fleischer, T.C.; Edoh, D.A. Antihyperglycaemic activity of ethanolic extract of the stem of Adenia lobata Engl. (Passifloraceae). Int. J. Pharm. Sci. Res. 2013, 4, 1370–1377. [Google Scholar]
- Tanaka, M.; Misawa, E.; Ito, Y.; Habara, N.; Nomaguchi, K.; Yamada, M.; Toida, T.; Hayasawa, H.; Takase, M.; Inagaki, M.; et al. Identification of Five Phytosterols from Aloe Vera Gel as Anti-diabetic Compounds. Biol. Pharm. Bull. 2006, 29, 1418–1422. [Google Scholar] [CrossRef] [Green Version]
- de Menezes Patrício Santos, C.C.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.C.; de Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef] [Green Version]
- Men’shchikova, E.B.; Lankin, V.Z.; Zenkov, N.K.; Bondar, I.A.; Krugovyh, N.F.; Trufakin, V.A. Okislitelnyi Stress: Prooksidanty i Antioksidanty; Slovo: Moskow, Russia, 2006; pp. 360–380. (In Russian) [Google Scholar]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Yu, Y.; Correll, P.H.; Vanden Heuvel, J.P. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: Evidence for a PPARg-dependent mechanism. Biochim. Biophys. Acta. 2002, 1581, 89–99. [Google Scholar] [CrossRef]
- Karakas, F.P.; Turker, A.U.; Karakas, A.; Mshvildadze, V. Cytotoxic, anti-inflammatory and antioxidant activities of four different extracts of Galega officinalis L (Goat’s rue). Trop. J. Pharm. Res. 2016, 15, 751–757. [Google Scholar] [CrossRef] [Green Version]







| Parameters | Groops | |||||
|---|---|---|---|---|---|---|
| C | C + G600 * | C + G1200 * | D | D + G600 * | D + G1200 * | |
| The number of islets of Langerhans on a standard area (N/10 mm2) | 15.91 ± 0.955 | 14.94 ± 0.945 | 15.65 ± 0.595 | 4.420 ± 0.743 a | 5.673 ± 0.631 a | 6.640 ± 0.605 ab |
| Islets area (μm2) | 10,440 ± 358.5 | 9785 ± 523.7 | 10,900 ± 388.2 | 4620 ± 497.0 a | 5354 ± 387.4 a | 6623 ± 331.2 abc |
| Islets diameter (μm) | 160.7 ± 3.814 | 152.6 ± 5.658 | 161.9 ± 5.548 | 106.5 ± 4.669 a | 113.7 ± 3.752 a | 119.7 ± 4.663 a |
| Islets volume (μm3) | 2.20 × 106 ± 1.52 × 105 | 1.92 × 106 ± 2.29 × 105 | 2.28 × 106 ± 2.38 × 105 | 6.58 × 105 ± 8.88 × 104 a | 7.88 × 105 ± 7.40 × 104 a | 9.28 × 105 ± 11.37 × 104 a |
| Number of β-cells/1000 μm² | 7.79 ± 0.280 | 7.18 ± 0.192 | 8.09 ± 0.308 | 4.74 ± 0.174 a | 5.12 ± 0.325 a | 5.62 ± 0.145 ab |
| Sample | TPC, mg GA/g | DPPH, μmol TE/g Sample | ATBS, μmol TE/g Sample | FRAP, μmol TE/g Sample |
|---|---|---|---|---|
| Galega officinalis extract | 28.77 ± 2.47 | 56.72 ± 1.52 | 114.73 ± 5.95 | 150.56 ± 2.76 |
| 50% water-ethanol extract of yacon | 92.30 ± 4.26 | 495.13 ± 17.64 | 728.4 ± 16.6 | 504.5 ± 6.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachkova, H.; Nagalievska, M.; Soliljak, Z.; Kanyuka, O.; Kucharska, A.Z.; Sokół-Łętowska, A.; Belonovskaya, E.; Buko, V.; Sybirna, N. Medicinal Plants Galega officinalis L. and Yacon Leaves as Potential Sources of Antidiabetic Drugs. Antioxidants 2021, 10, 1362. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10091362
Hachkova H, Nagalievska M, Soliljak Z, Kanyuka O, Kucharska AZ, Sokół-Łętowska A, Belonovskaya E, Buko V, Sybirna N. Medicinal Plants Galega officinalis L. and Yacon Leaves as Potential Sources of Antidiabetic Drugs. Antioxidants. 2021; 10(9):1362. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10091362
Chicago/Turabian StyleHachkova, Halyna, Mariia Nagalievska, Zoriana Soliljak, Olena Kanyuka, Alicja Zofia Kucharska, Anna Sokół-Łętowska, Elena Belonovskaya, Vyacheslav Buko, and Nataliia Sybirna. 2021. "Medicinal Plants Galega officinalis L. and Yacon Leaves as Potential Sources of Antidiabetic Drugs" Antioxidants 10, no. 9: 1362. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10091362