Comprehensive Metabolomics and Machine Learning Identify Profound Oxidative Stress and Inflammation Signatures in Hypertensive Patients with Obstructive Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
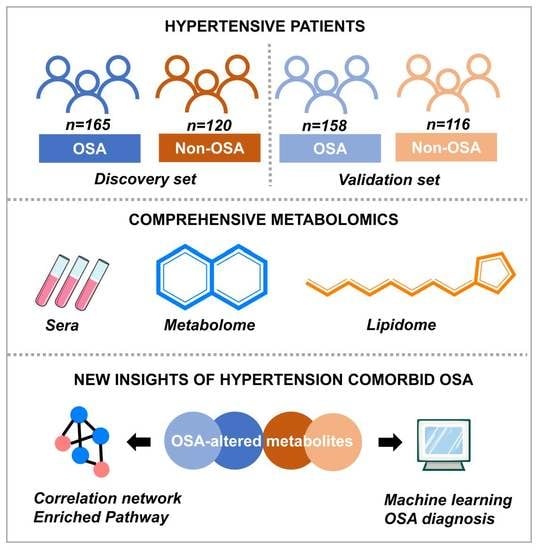
2.1. Subjects, Clinical Assessment, and Study Design
2.2. Data Collection and Clinical Laboratory Tests
2.3. Serum Metabolome and Lipidome Analyses
2.4. Metabolic Data Processing and Pathway Analysis
2.5. Important Metabolic Feature Identification and Diagnosis Model Performances
2.6. Statistical Analysis
3. Results
3.1. Participant Clinical Characteristics
3.2. Metabolic Phenotypes of Hypertensive Patients with and without OSA
3.3. Identification of a Metabolic Fingerprint Specific for Hypertension Comorbid OSA
3.4. Metabolic Pathway Enrichment and Functional Analysis
3.5. Metabolites Association with PSG Measures, Blood Pressure, and Cardiovascular Risk Factors
3.6. Multi-Metabolites Model for Diagnosis of OSA in Hypertensive Individuals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive sleep apnea and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Furlan, S.F.; Braz, C.V.; Lorenzi-Filho, G.; Drager, L.F. Management of hypertension in obstructive sleep apnea. Curr. Cardiol. Rep. 2015, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Tokunou, T.; Ando, S.I. Recent advances in the management of secondary hypertension-obstructive sleep apnea. Hypertens. Res. 2020, 43, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.P.; Drager, L.F.; Gonzaga, C.C.; Sousa, M.G.; de Paula, L.K.; Amaro, A.C.; Amodeo, C.; Bortolotto, L.A.; Krieger, E.M.; Bradley, T.D.; et al. Obstructive sleep apnea: The most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011, 58, 811–817. [Google Scholar] [CrossRef]
- Sanchez-de-la-Torre, M.; Khalyfa, A.; Sanchez-de-la-Torre, A.; Martinez-Alonso, M.; Martinez-Garcia, M.A.; Barcelo, A.; Lloberes, P.; Campos-Rodriguez, F.; Capote, F.; Diaz-de-Atauri, M.J.; et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: Blood pressure response to continuous positive airway pressure treatment. J. Am. Coll. Cardiol. 2015, 66, 1023–1032. [Google Scholar] [CrossRef]
- Khan, S.U.; Duran, C.A.; Rahman, H.; Lekkala, M.; Saleem, M.A.; Kaluski, E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur. Heart J. 2018, 39, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Sethi, R.; Li, R.; Ho, H.H.; Hein, T.; Jim, M.H.; Loo, G.; Koo, C.Y.; Gao, X.F.; Chandra, S.; et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef]
- Sanchez-de-la-Torre, M.; Sanchez-de-la-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- Pengo, M.F.; Javaheri, S.; Sanchez-de-la-Torre, M.; Schwarz, E.I. What cardiologists should know about sleep. Eur. Heart J. 2022, 43, 2911–2913. [Google Scholar] [CrossRef]
- Konecny, T.; Kara, T.; Somers, V.K. Obstructive sleep apnea and hypertension: An update. Hypertension 2014, 63, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Wang, L.; Zhou, Y. Hypertension and obstructive sleep apnea. Hypertens. Res. 2016, 39, 391–395. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; He, F.; Calhoun, S.L.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Association of pediatric obstructive sleep apnea with elevated blood pressure and orthostatic hypertension in adolescence. JAMA Cardiol. 2021, 6, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Nowak, N.; Engler, A.; Thiel, S.; Stoberl, A.S.; Sinues, P.; Zenobi, R.; Kohler, M. Validation of breath biomarkers for obstructive sleep apnea. Sleep Med. 2021, 85, 75–86. [Google Scholar] [CrossRef]
- Pirhaji, L.; Milani, P.; Leidl, M.; Curran, T.; Avila-Pacheco, J.; Clish, C.B.; White, F.M.; Saghatelian, A.; Fraenkel, E. Revealing disease-associated pathways by network integration of untargeted metabolomics. Nat. Methods 2016, 13, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Greco, M.; Toraldo, D.M.; Arigliani, M.; Maffia, M.; De Benedetto, M. A review of the “OMICS” for management of patients with obstructive sleep apnoea. Acta Otorhinolaryngol. Ital. 2020, 40, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Salihovic, S.; Fall, T.; Hammar, U.; Ingelsson, E.; Arnlov, J.; Lind, L.; Sundstrom, J. Global plasma metabolomics to identify potential biomarkers of blood pressure progression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e227–e237. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Graham, D.; Kastenmuller, G.; Alharbi, N.H.; Alsanosi, S.M.; McBride, M.; Mangino, M.; Titcombe, P.; Shin, S.Y.; Psatha, M.; et al. Metabolomic identification of a novel pathway of blood pressure regulation involving hexadecanedioate. Hypertension 2015, 66, 422–429. [Google Scholar] [CrossRef]
- Ussher, J.R.; Elmariah, S.; Gerszten, R.E.; Dyck, J.R. The Emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J. Am. Coll. Cardiol. 2016, 68, 2850–2870. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.J.; Collins, K.J.; Dennison, H.C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Mediano, O.; Gonzalez, M.N.; Montserrat, J.M.; Alonso-Alvarez, M.L.; Almendros, I.; Alonso-Fernandez, A.; Barbe, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International consensus document on obstructive sleep apnea. Arch. Bronconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef]
- Fatureto-Borges, F.; Jenner, R.; Costa-Hong, V.; Lopes, H.F.; Teixeira, S.H.; Marum, E.; Giorgi, D.; Consolim-Colombo, F.M.; Bortolotto, L.A.; Lorenzi-Filho, G.; et al. Does obstructive Sleep Apnea Influence Blood Pressure and Arterial Stiffness in Response to Antihypertensive Treatment? Hypertension 2018, 72, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, X.; Chen, W.; Zhong, L.; Cui, M. Comprehensive plasma metabolomic and lipidomic analyses reveal potential biomarkers for heart failure. Mol. Cell Biochem. 2021, 476, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, L.; Chen, L.; Liu, S.; Zhong, L.; Cui, M. Comprehensive metabolomic and proteomic analyses reveal candidate biomarkers and related metabolic networks in atrial fibrillation. Metabolomics 2019, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Li, L.; Zhang, Y.; Lu, J.; Wang, X.; Song, X.; Liu, J.; Li, K. Machine learning identifies metabolic signatuRes. that predict the risk of recurrent angina in remitted patients after percutaneous coronary intervention: A multicenter prospective cohort study. Adv. Sci. 2021, 8, 2003893. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Miao, A.; Yang, C.; Huang, C.; Chen, H.; Jiang, Y.; Deng, C.; Sun, N. Precise detection of cataracts with specific high-risk factors by layered binary co-ionizers assisted aqueous humor metabolic analysis. Adv. Sci. 2022, 9, e2105905. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Yuan, Y.D.; Nawaz, W.; Ze, H.; Zhuo, C.X.; Talal, B.; Taleb, A.; Mais, E.; Qilong, D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic. Res. 2017, 51, 428–438. [Google Scholar] [CrossRef]
- Stanek, A.; Brozyna-Tkaczyk, K.; Myslinski, W. Oxidative stress markers among obstructive sleep apnea patients. Oxid. Med. Cell. Longev. 2021, 2021, 9681595. [Google Scholar] [CrossRef]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The role of taurine in mitochondria health: More than just an antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Liu, Y.; Gao, J.; Tao, J. The molecular targets of taurine confer anti-hyperlipidemic effects. Life Sci. 2021, 278, 119579. [Google Scholar] [CrossRef]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The anti-inflammatory effect of taurine on cardiovascular disease. Nutrients 2020, 12, 2847. [Google Scholar] [CrossRef]
- Holecek, M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Durante, W. The emerging role of L-glutamine in cardiovascular health and disease. Nutrients 2019, 11, 2092. [Google Scholar] [CrossRef]
- Drager, L.F.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: An emerging risk factor for atherosclerosis. Chest 2011, 140, 534–542. [Google Scholar] [CrossRef]
- Guralnick, A.S. Obstructive sleep apnea: Incidence and impact on hypertension? Curr. Cardiol. Rep. 2013, 15, 415. [Google Scholar] [CrossRef]
- Shin, H.W.; Cho, K.; Rhee, C.S.; Hong, I.H.; Cho, S.H.; Kim, S.W.; Kim, J.; So, D.; Cho, J.Y.; Park, J.W. Urine 5-eicosatetraenoic acids as diagnostic markers for obstructive sleep apnea. Antioxidants 2021, 10, 1242. [Google Scholar] [CrossRef]
- Lebkuchen, A.; Carvalho, V.M.; Venturini, G.; Salgueiro, J.S.; Freitas, L.S.; Dellavance, A.; Martins, F.C.; Lorenzi-Filho, G.; Cardozo, K.; Drager, L.F. Metabolomic and lipidomic profile in men with obstructive sleep apnoea: Implications for diagnosis and biomarkers of cardiovascular risk. Sci. Rep. 2018, 8, 11270. [Google Scholar] [CrossRef] [Green Version]
- Pak, V.M.; Russell, K.; Shi, Z.; Zhang, Q.; Cox, J.; Uppal, K.; Yu, T.; Hertzberg, V.; Liu, K.; Ioachimescu, O.C.; et al. Sphinganine is associated with 24-h MAP in the non-sleepy with OSA. Metabolomics 2022, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Micova, P.; Hahnova, K.; Hlavackova, M.; Elsnicova, B.; Chytilova, A.; Holzerova, K.; Zurmanova, J.; Neckar, J.; Kolar, F.; Novakova, O.; et al. Chronic intermittent hypoxia affects the cytosolic phospholipase A2alpha/cyclooxygenase 2 pathway via beta2-adrenoceptor-mediated ERK/p38 stimulation. Mol. Cell Biochem. 2016, 423, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Little, P.J.; Ta, H.T.; Xu, S.; Kamato, D. Lysophosphatidic acid and its receptors: Pharmacology and therapeutic potential in atherosclerosis and vascular disease. Pharmacol. Ther. 2019, 204, 107404. [Google Scholar] [CrossRef]
- Oestvang, J.; Anthonsen, M.W.; Johansen, B. LysoPC and PAF trigger arachidonic acid release by divergent signaling mechanisms in Monocytes. J. Lipids 2011, 2011, 532145. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Nakamura, T.; Ohan, J.; Leseche, G.; Tedgui, A.; Maclouf, J.; Murphy, R.C. The relationship of hydroxyeicosatetraenoic acids and F2-isoprostanes to plaque instability in human carotid atherosclerosis. J. Clin. Investig. 1999, 103, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Zu, L.; Guo, G.; Zhou, B.; Gao, W. Relationship between metabolites of arachidonic acid and prognosis in patients with acute coronary syndrome. Thromb. Res. 2016, 144, 192–201. [Google Scholar] [CrossRef]
- Cowie, M.R.; Linz, D.; Redline, S.; Somers, V.K.; Simonds, A.K. Sleep disordered breathing and cardiovascular disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 608–624. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, X.; Jia, W.; Yin, S. Chromatography/mass spectrometry-based biomarkers in the field of obstructive sleep apnea. Medicine 2015, 94, e1541. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xu, H.; Yi, H.; Guan, J.; Yin, S. Metabolomics and microbiome profiling as biomarkers in obstructive sleep apnoea: A comprehensive review. Eur. Respir. Rev. 2021, 30, 200220. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gileles-Hillel, A.; Gozal, D. The challenges of precision medicine in obstructive sleep apnea. Sleep Med. Clin. 2016, 11, 213–226. [Google Scholar] [CrossRef]
- Weng, S.F.; Reps, J.; Kai, J.; Garibaldi, J.M.; Qureshi, N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS ONE 2017, 12, e174944. [Google Scholar] [CrossRef] [PubMed]
- Olkowicz, M.; Czyzynska-Cichon, I.; Szupryczynska, N.; Kostogrys, R.B.; Kochan, Z.; Debski, J.; Dadlez, M.; Chlopicki, S.; Smolenski, R.T. Multi-omic signatuRes. of atherogenic dyslipidaemia: Pre-clinical target identification and validation in humans. J. Transl. Med. 2021, 19, 6. [Google Scholar] [CrossRef] [PubMed]






| Discovery Set | Validation Set | |||||
|---|---|---|---|---|---|---|
| Variables | OSA (n = 165) | Non-OSA (n = 120) | p | OSA (n = 158) | Non-OSA (n = 116) | p |
| General characteristics | ||||||
| Ages | 46.2 ± 13.7 | 43.2 ± 12.4 | 0.059 | 45.67 ± 12.38 | 45.28 ± 11.73 | 0.8 |
| Male, n (%) | 120, (74.53%) | 86, (71.67) | 0.59 | 115, (77.18%) | 81, (69.83%) | 0.18 |
| Current smoker, n (%) | 108, (67.08%) | 77, (64.17%) | 0.61 | 100, (67.11%) | 73, (63.79%) | 0.57 |
| Alcohol intake > 10 g/day, n (%) | 99, (61.49%) | 71, (59.71%) | 0.69 | 97, (65.10%) | 72, (62.07%) | 0.61 |
| Diabetes mellitus, n (%) | 43, (26.88%) | 33, (27.50%) | 0.91 | 44, (27.85%) | 29, (25.00%) | 0.60 |
| Sleeping pills users, n (%) | 8, (4.85%) | 5, (4.17%) | 0.79 | 11, (6.96%) | 4, (3.46%) | 0.21 |
| Neck circumference (cm) | 42.46 ± 4.10 | 40.64 ± 4.13 | 0.006 | 42.47 ± 3.97 | 40.95 ± 3.95 | 0.034 |
| Waist circumference (cm) | 103.35 ± 11.43 | 95.52 ± 13.02 | <0.0001 | 102.93 ± 10.38 | 97.01 ± 10.44 | 0.0004 |
| Hip circumference (cm) | 108.40 ± 8.96 | 104.65 ± 11.90 | 0.026 | 107.89 ± 10.13 | 103.63 ± 8.44 | 0.013 |
| Waist-to-Hip ratio | 0.96 ± 0.067 | 0.92 ± 0.069 | 0.0002 | 0.96 ± 0.052 | 0.93 ± 0.043 | 0.0043 |
| BMI (kg/m2) | 29.93 ± 4.48 | 27.26 ± 4.11 | <0.0001 | 29.47 ± 4.67 | 27.52 ± 4.92 | 0.0016 |
| Heart rate (times/min) | 73.03 ± 9.47 | 72.30 ± 8.92 | 0.54 | 72.10 ± 9.64 | 73.86 ± 9.84 | 0.17 |
| BP parameters | ||||||
| Mean office SBP (mmHg) | 141.65 ± 20.25 | 137.78 ± 18.08 | 0.11 | 144.31 ± 19.90 | 142.29 ± 19.24 | 0.44 |
| Mean office DBP (mmHg) | 91.58 ± 15.79 | 90.42 ± 12.95 | 0.53 | 92.89 ± 15.59 | 91.85 ± 15.09 | 0.61 |
| 24-h SBP (mmHg) | 134.05 ± 16.55 | 130.20 ± 13.05 | 0.069 | 134.80 ± 14.13 | 131.71 ± 14.90 | 0.14 |
| 24-h DBP (mmHg) | 85.83 ± 11.79 | 84.72 ± 9.86 | 0.47 | 87.05 ± 10.07 | 84.57 ± 10.99 | 0.11 |
| Day-time SBP (mmHg) | 137.43 ± 16.54 | 134.27 ± 13.36 | 0.14 | 137.95 ± 14.39 | 133.84 ± 15.17 | 0.057 |
| Day-time DBP (mmHg) | 88.03 ± 11.82 | 87.76 ± 9.60 | 0.86 | 88.86 ± 9.78 | 86.08 ± 10.59 | 0.061 |
| Night-time SBP (mmHg) | 128.50 ± 18.86 | 123.51 ± 16.20 | 0.045 | 130.98 ± 16.98 | 126.06 ± 16.04 | 0.044 |
| Night-time DBP (mmHg) | 82.05 ± 13.84 | 78.54 ± 10.41 | 0.042 | 84.43 ± 12.32 | 80.11 ± 11.33 | 0.014 |
| Non-dipper, n (%) | 75, (45.45%) | 46, (38.33%) | 0.23 | 77, (48.73%) | 48, (41.38%) | 0.23 |
| Laboratory tests | ||||||
| Fasting blood glucose (mmol/L) | 6.05 ± 1.39 | 5.79 ± 1.41 | 0.14 | 6.27 ± 2.14 | 5.91 ± 1.66 | 0.17 |
| LDL-C (mmol/L) | 3.02 ± 0.98 | 3.08 ± 0.85 | 0.65 | 3.06 ± 0.83 | 3.05 ± 0.84 | 0.94 |
| TC (mmol/L) | 4.93 ± 1.05 | 4.87 ± 1.08 | 0.65 | 4.95 ± 0.99 | 4.90 ± 0.89 | 0.71 |
| TG (mmol/L) | 1.78 [1.39, 2.49] | 1.43 [1.07, 2.27] | 0.002 | 1.86 [1.21, 2.83] | 1.53 [1.09, 2.25] | 0.046 |
| HDL-C (mmol/L) | 1.11 ± 0.24 | 1.25 ± 0.26 | <0.0001 | 1.17 ± 0.24 | 1.26 ± 0.38 | 0.043 |
| ALT (mmol/L) | 28.98 ± 14.41 | 28.25 ± 15.81 | 0.77 | 30.56 ± 13.03 | 28.87 ± 14.38 | 0.55 |
| Non-HDL (mmol/L) | 3.74 ± 1.13 | 3.61 ± 1.02 | 0.35 | 3.57 ± 1.29 | 3.42 ± 1.25 | 0.34 |
| AST (mmol/L) | 26.39 ± 10.25 | 25.05 ± 9.26 | 0.39 | 26.81 ± 12.45 | 24.48 ± 11.48 | 0.33 |
| Sleep parameters | ||||||
| AHI(events/h) | 39.66 ± 22.41 | 6.42 ± 5.29 | <0.0001 | 35.94 ± 18.77 | 8.63 ± 3.85 | <0.0001 |
| LaSO2(%) | 78.25 ± 7.79 | 87.94 ± 5.62 | <0.0001 | 76.82 ± 10.84 | 84.88 ± 10.91 | <0.0001 |
| MSaO2(%) | 93.58 ± 2.16 | 95.61 ± 1.57 | <0.0001 | 93.81 ± 2.09 | 94.90 ± 1.62 | <0.0001 |
| MAD(s) | 26.19 ± 5.66 | 22.02 ± 8.01 | 0.00015 | 25.21 ± 6.08 | 22.72 ± 6.66 | 0.035 |
| CT90(%) | 4.12 [1.36, 12.80] | 0.10 [0.00, 0.31] | <0.0001 | 5.70 [1.74, 16.29] | 0.20 [0.00, 1.00] | <0.0001 |
| ODI(events/h) | 31.81 [19.98, 47.46] | 4.65 [1.14, 8.75] | <0.0001 | 30.90 [19.34, 43.95] | 8.65 [4.84, 11.86] | <0.0001 |
| ArI(events/h) | 20.85 [10.81, 34.13] | 8.60 [4.51, 12.93] | <0.0001 | 18.10 [5.03, 32.35] | 10.40 [4.08, 27.90] | 0.00096 |
| ESS score | 8.75 ± 5.06 | 7.28 ± 5.12 | 0.018 | 9.49 ± 5.01 | 8.04 ± 4.88 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Sun, H.; Du, Y.; Li, L.; Lv, Q.; Yu, H.; Li, F.; Wang, Y.; Jiao, X.; Hu, C.; et al. Comprehensive Metabolomics and Machine Learning Identify Profound Oxidative Stress and Inflammation Signatures in Hypertensive Patients with Obstructive Sleep Apnea. Antioxidants 2022, 11, 1946. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11101946
Du Z, Sun H, Du Y, Li L, Lv Q, Yu H, Li F, Wang Y, Jiao X, Hu C, et al. Comprehensive Metabolomics and Machine Learning Identify Profound Oxidative Stress and Inflammation Signatures in Hypertensive Patients with Obstructive Sleep Apnea. Antioxidants. 2022; 11(10):1946. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11101946
Chicago/Turabian StyleDu, Zhiyong, Haili Sun, Yunhui Du, Linyi Li, Qianwen Lv, Huahui Yu, Fan Li, Yu Wang, Xiaolu Jiao, Chaowei Hu, and et al. 2022. "Comprehensive Metabolomics and Machine Learning Identify Profound Oxidative Stress and Inflammation Signatures in Hypertensive Patients with Obstructive Sleep Apnea" Antioxidants 11, no. 10: 1946. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11101946