Salicornia ramosissima: A New Green Cosmetic Ingredient with Promising Skin Effects
Abstract
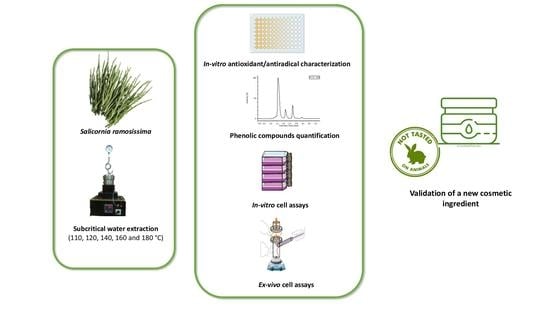
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample
2.3. Subcritical Water Extraction (SWE) of S. ramosissima
2.4. Quantification of the Bioactive Compounds
Determination of Total Polyphenols Content
2.5. In Vitro Antioxidant/Antiradical Activities
2.5.1. Ferric Reducing Antioxidant Power Assay
2.5.2. ABTS•+ Radical Scavenging Activity Assay
2.5.3. DPPH• Radical Scavenging Activity Assay
2.6. Reactive Oxygen Species Scavenging Capacity Assays
2.6.1. Superoxide Radical Scavenging Assay
2.6.2. Hypochlorous Acid scavenging Assay
2.7. Phytochemical Profile of S. ramosissima Extracts
2.7.1. Preparation of Polyphenols Standards
2.7.2. High Performance Liquid Chromatography (HPLC) System
2.7.3. HPLC Chromatographic Conditions
2.8. Cell Viability Assays
2.9. Ex Vivo Permeation Assay in Franz Diffusion Cell
2.10. Enzymatic Assays
2.10.1. Human Neutrophil Elastase Inhibition Assay
2.10.2. Hyaluronidase Inhibition Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield, TPC and Antioxidant/Antiradical Activities
3.2. Phytochemical Profile of S. ramosissima Extracts
3.3. Reactive Oxygen Species Scavenging Capacity Assays
3.4. Cellular Viability Assays
3.5. Ex Vivo Permeation Assays in Franz Diffusion Cells
3.6. Hyaluronidase and Elastase Inhibition Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.; García-Oliveira, P.; Prieto, M.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. A potential valorization strategy of wine industry by-products and their application in cosmetics—Case study: Grape pomace and grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, A.; Kaur, J.; Kumari, S.; Garg, M.; Sindhu, R.K.; Rahman, M.H.; Akhtar, M.F.; Tagde, P.; Najda, A. Bioactive-based cosmeceuticals: An update on emerging trends. Molecules 2022, 27, 828. [Google Scholar] [CrossRef] [PubMed]
- Kadereit, G.; Ball, P.; Beer, S.; Mucina, L.; Sokoloff, D.; Teege, P.; Yaprak, A.E.; Freitag, H. A taxonomic nightmare comes true: Phylogeny and biogeography of glassworts (Salicornia L., Chenopodiaceae). Taxon 2007, 56, 1143–1170. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional characterization and storage ability of Salicornia ramosissima and Sarcocornia perennis for fresh vegetable salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Jang, H.-S.; Kim, K.-R.; Choi, S.-W.; Woo, M.-H.; Choi, J.-H. Antioxidant and antithrombus activities of enzyme-treated Salicornia herbacea extracts. Ann. Nutr. Metab. 2007, 51, 119–125. [Google Scholar] [CrossRef]
- Isca, V.M.; Seca, A.M.; Pinto, D.C.; Silva, H.; Silva, A.M. Lipophilic profile of the edible halophyte Salicornia ramosissima. Food Chem. 2014, 165, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- de Jesús Cortés-Sánchez, A.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: A review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.; Vian, M.; Cavalluzzi, M.M.; Franchini, C.; Corbo, F.; Lentini, G.; Chemat, F. Ultrasound and deep eutectic solvents: An efficient combination to tune the mechanism of steviol glycosides extraction. Ultrason. Sonochemistry 2020, 69, 105255. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Garcia, S.L.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Cheigh, C.I.; Chung, E.Y.; Chung, M.S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Castroviejo, S.; Muñoz Garmendia, F. Flora ibérica: Plantas vasculares de la Península Ibérica e Islas Baleares: Vol. IX. Flora Ibér. 2015, 9, 1–612. [Google Scholar]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Linares, I.B.; Mašković, P. Characterisation of ginger extracts obtained by subcritical water. J. Supercrit. Fluids 2017, 123, 92–100. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Baptista, P.; Ferreira, I.C.F.R. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 2007, 45, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel strong scavengers of reactive oxygen and nitrogen species. Bioorganic Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Rodrigues, F.; Dorosh, O.; Pinto, D.; Costa, P.C.; Švarc-Gajić, J.; Delerue-Matos, C. Vine-canes as a source of value-added compounds for cosmetic formulations. Molecules 2020, 25, 2969. [Google Scholar] [CrossRef]
- Rodrigues, F.; Alves, A.C.; Nunes, C.; Sarmento, B.; Amaral, M.H.; Reis, S.; Oliveira, M.B.P. Permeation of topically applied caffeine from a food by—Product in cosmetic formulations: Is nanoscale in vitro approach an option? Int. J. Pharm. 2016, 513, 496–503. [Google Scholar] [CrossRef]
- Silva, A.M.; Garcia, J.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Eco-friendly insights on kiwiberry leaves valorization through in-vitro and in-vivo studies. Ind. Crops Prod. 2022, 184, 115090. [Google Scholar] [CrossRef]
- Nema, N.K.; Maity, N.; Sarkar, B.; Mukherjee, P.K. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch. Dermatol. Res. 2011, 303, 247–252. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Pinto, D.; Reis, J.; Silva, A.M.; Salazar, M.; Dall’Acqua, S.; Delerue-Matos, C.; Rodrigues, F. Valorisation of Salicornia ramosissima biowaste by a green approach–An optimizing study using response surface methodology. Sustain. Chem. Pharm. 2021, 24, 100548. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Ciulu, M.; Quirantes-Piné, R.; Spano, N.; Sanna, G.; Borrás-Linares, I.; Segura-Carretero, A. Evaluation of new extraction approaches to obtain phenolic compound-rich extracts from Stevia rebaudiana Bertoni leaves. Ind. Crops Prod. 2017, 108, 106–112. [Google Scholar] [CrossRef]
- Veličković, V.; Đurović, S.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Vujić, J.; Trifunović, S.; Mašković, P.Z. Application of conventional and non-conventional extraction approaches for extraction of Erica carnea L.: Chemical profile and biological activity of obtained extracts. J. Supercrit. Fluids 2017, 128, 331–337. [Google Scholar] [CrossRef]
- Rodrigues, L.G.G.; Mazzutti, S.; Siddique, I.; da Silva, M.; Vitali, L.; Ferreira, S.R.S. Subcritical water extraction and microwave-assisted extraction applied for the recovery of bioactive components from Chaya (Cnidoscolus aconitifolius Mill.). J. Supercrit. Fluids 2020, 165, 104976. [Google Scholar] [CrossRef]
- Kheirkhah, H.; Baroutian, S.; Quek, S.Y. Evaluation of bioactive compounds extracted from Hayward kiwifruit pomace by subcritical water extraction. Food Bioprod. Process. 2019, 115, 143–153. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process.-Process Intensif. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, X.; Zhang, C.; Lu, Y.; Wei, D. Protective effects of hyperoside (quercetin-3-o-galactoside) to PC12 cells against cytotoxicity induced by hydrogen peroxide and tert-butyl hydroperoxide. Biomed. Pharmacother. 2005, 59, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Stuper-Szablewska, K. Phenolic acids used in the cosmetics industry as natural antioxidants. EJMT 2019, 4, 24–32. [Google Scholar]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef]
- Shin, J.Y.; Park, J.H.; Che, D.N.; Kang, H.J.; Cho, B.O.; Lim, Y.T.; Jang, S.I. Protective effects of halophyte complex extract against UVB-induced damage in human keratinocytes and the skin of hairless mice. Exp. Ther. Med. 2021, 22, 682. [Google Scholar] [CrossRef]
- Jo, Y.J.; Yoo, D.H.; Lee, I.C.; Lee, J.; Jeong, H.S. Antioxidant and Skin Whitening Activities of Sub-and Super-Critical Water Treated Rutin. Molecules 2022, 27, 5441. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Natural exogenous antioxidant defense against changes in human skin fibroblast proteome disturbed by UVA radiation. Oxidative Med. Cell. Longev. 2020, 2020, 3216415. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
- Esposito, L.; Barbosa, A.I.; Moniz, T.; Costa Lima, S.; Costa, P.; Celia, C.; Reis, S. Design and characterization of sodium alginate and poly (vinyl) alcohol hydrogels for enhanced skin delivery of quercetin. Pharmaceutics 2020, 12, 1149. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.-G.; Byun, S. Quercetin directly targets JAK2 and PKCδ and prevents UV-induced photoaging in human skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmowafy, M.; Musa, A.; Alnusaire, T.S.; Shalaby, K.; Fouda, M.M.; Salama, A.; Al-Sanea, M.M.; Abdelgawad, M.A.; Gamal, M.; Fouad, S.A. Olive oil/pluronic oleogels for skin delivery of quercetin: In vitro characterization and ex vivo skin permeability. Polymers 2021, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.R.; Selvakumar, G.P.; Sivakamasundari, R.I. Effects of syringic acid on chronic MPTP/probenecid induced motor dysfunction, dopaminergic markers expression and neuroinflammation in C57BL/6 mice. Biomed. Aging Pathol. 2014, 4, 95–104. [Google Scholar] [CrossRef]
- Ding, S.K.; Wang, L.X.; Guo, L.S.; Luo, P.; Du, J.J.; Zhao, Z.L.; Wang, G.G. Syringic acid inhibits apoptosis pathways via downregulation of p38MAPK and JNK signaling pathways in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Mol. Med. Rep. 2017, 16, 2290–2294. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.J.; Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Song, K.-M.; Chang, P.-S.; Jeong, C.-H.; Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018, 154, 435–445. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef] [Green Version]
- Eun, C.-H.; Kang, M.-S.; Kim, I.-J. Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity. Appl. Sci. 2020, 10, 4838. [Google Scholar] [CrossRef]
- El-Nashar, H.A.; El-Labbad, E.M.; Al-Azzawi, M.A.; Ashmawy, N.S. A New Xanthone Glycoside from Mangifera indica L.: Physicochemical Properties and In Vitro Anti-Skin Aging Activities. Molecules 2022, 27, 2609. [Google Scholar] [CrossRef]
- Ahn, B.-K.; Kim, R.; Choi, D.; Kim, Y.-S. Effect of Salicornia bigelovii extract on the activities of whitening and anti-wrinkle. Appl. Chem. Eng. 2011, 22, 56–60. [Google Scholar]

| Extraction Temperature (°C) | Extraction Yield (%) | TPC (mg GAE/g dw) | FRAP (µmol FSE/g dw) | ABTS•+ (mg AAE/g dw) | DPPH (mg TE/g dw) |
|---|---|---|---|---|---|
| 110 | 21.27 ± 0.14 | 22.41 ± 0.04 a | 193.32 ± 7.72 c | 31.86 ± 3.28 b | 10.17 ± 1.67 c |
| 120 | 21.34 ± 0.07 | 23.86 ± 2.05 a | 144.37 ± 10.05 d | 28,23 ± 0.92 b | 19.93 ± 2.19 b,c |
| 140 | 21.65 ± 0.24 | 25.66 ± 2.22 a | 422.57 ± 5.67 a | 32.72 ± 3.27 a,b | 23.12 ± 1.77 a,b,c |
| 160 | 21.42 ± 0.01 | 14.55 ± 2.04 b | 283.63 ± 1.17 b | 39.50 ± 3.95 a | 34.41 ± 3.63 a |
| 180 | 21.36 ± 0.05 | 21.53 ± 2.08 a | 264.95 ± 18.79 b | 34.97 ± 4.48 a,b | 28.14 ± 0.81 a,b |
| Compounds | SWE 110 °C (mg/100 g dw) | SWE 120 °C (mg/100 g dw) | SWE 140 °C (mg/100 g dw) | SWE 160 °C (mg/100 g dw) | SWE 180 °C (mg/100 g dw) |
|---|---|---|---|---|---|
| Phenolic acids | |||||
| Gallic acid | 37.88 ± 1.89 | 39.40 ± 1.97 | 42.90 ± 2.15 | 3.28 ± 0.16 | 94.01 ± 4.70 |
| Protocatechuic acid | 80.82 ± 4.04 | 197.64 ± 9.88 | 241.64 ± 12.08 | 139.16 ± 6.96 | 302.06 ± 15.10 |
| Neochlorogenic acid | 5.33 ± 0.27 | 40.72 ± 2.04 | 39.78 ± 1.99 | 39.10 ± 1.95 | 92.32 ± 4.62 |
| Vanillic acid | 12.24 ± 0.61 | 18.25 ± 0.91 | 36.95 ± 1.85 | 37.27 ± 1.86 | 36.47 ± 1.82 |
| Caffeic acid | <LOQ | 6.72 ± 0.34 | 9.69 ± 0.48 | 32.45 ± 1.62 | 51.83 ± 2.59 |
| Syringic acid | 4.11 ± 0.21 | 10.05 ± 0.50 | 10.56 ± 0.53 | 12.47 ± 0.62 | 12.00 ± 0.60 |
| Caftaric acid | 7.46 ± 0.37 | 131.11 ± 6.56 | 87.44 ± 4.37 | 78.08 ± 3.90 | 108.14 ± 5.41 |
| Chlorogenic acid | 135.35 ± 6.77 | 326.52 ± 16.33 | 270.77 ± 13.54 | 270.11 ± 13.51 | 238.37 ± 11.92 |
| 4-O-caffeyolquinic acid | 11.55 ± 0.58 | 9.23 ± 0.46 | 11.91 ± 0.60 | 129.63 ± 6.48 | 105.47 ± 5.27 |
| p-Coumaric acid | 16.27 ± 0.81 | 31.16 ± 1.56 | 29.94 ± 1.50 | 23.70 ± 1.19 | <LOD |
| Ferulic acid | 1.30 ± 0.06 | <LOD | <LOQ | <LOQ | <LOQ |
| Sinapic acid | <LOQ | ND | <LOQ | <LOQ | <LOQ |
| 3.5-di-caffeoylquinic acid | <LOQ | <LOQ | 8.84 ± 0.44 | 9.46 ± 0.47 | <LOQ |
| Ellagic acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOD |
| 4.5-di-O-caffeoylquinic acid | 4.42 ± 0.22 | 7.29 ± 0.36 | 7.26 ± 0.36 | 11.35 ± 0.57 | 14.09 ± 0.70 |
| Cinnamic acid | ND | ND | ND | ND | ND |
| ∑Phenolic acids | 316.72 | 818.10 | 797.70 | 786.07 | 1054.77 |
| Flavanols | |||||
| Catechin | 277.13 ± 13.86 | 475.49 ± 23.77 | 359.43 ± 17.97 | 169.61 ± 8.48 | 519.93 ± 26.00 |
| Epicatechin | ND | ND | 49.61 ± 2.48 | 12.29 ± 0.61 | ND |
| ∑Flavanols | 277.13 | 475.49 | 409.04 | 181.90 | 519.93 |
| Flavanones | |||||
| Naringin | 3.36 ± 0.17 | 9.80 ± 0.49 | 3.26 ± 0.16 | 7.65 ± 0.38 | 7.84 ± 0.39 |
| Naringenin | ND | ND | ND | <LOD | ND |
| ∑Flavanones | 3.36 | 9.80 | 3.26 | 7.65 | 7.84 |
| Flavonols | |||||
| Quercetin-3-O-galactoside | 10.76 ± 0.54 | 23.51 ± 1.18 | 18.66 ± 0.93 | 51.64 ± 2.58 | 17.01 ± 0.85 |
| Rutin | ND | ND | <LOQ | <LOQ | ND |
| Myricetin | 14.20 ± 0.71 | 26.73 ± 1.34 | <LOQ | <LOD | <LOQ |
| Kaempferol-3-O-glucoside | ND | <LOQ | <LOQ | <LOQ | <LOD |
| Kaempferol-3-O-rutinoside | 3.17 ± 0.16 | <LOQ | <LOQ | 2.73 ± 0.14 | ND |
| Quercetin | <LOD | <LOQ | <LOQ | <LOQ | ND |
| Tiliroside | ND | ND | <LOQ | <LOQ | ND |
| Kaempferol | <LOQ | <LOD | <LOD | <LOQ | <LOD |
| Quercetin-3-O-glucopyranoside | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Isorhamnetin-3-O-glucoside | ND | ND | ND | ND | ND |
| Isorhamnetin-3-O-rutinoside | <LOD | <LOQ | <LOQ | 4.97 ± 0.25 | 9.14 ± 0.46 |
| ∑Flavonols | 28.13 | 50.24 | 18.66 | 59.34 | 26.15 |
| Flavones | |||||
| Apigenin | ND | <LOD | <LOD | ND | <LOD |
| Chrysin | <LOD | <LOD | ND | ND | <LOD |
| Quercitrin | ND | ND | ND | ND | ND |
| ∑Flavones | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Others | |||||
| Phloridzin | 6.88 ± 0.34 | 16.77 ± 0.84 | 19.47 ± 0.97 | 26.89 ± 1.34 | 21.80 ± 1.09 |
| Phloretin | <LOQ | <LOQ | <LOQ | <LOQ | <LOD |
| Resveratrol | ND | ND | ND | ND | ND |
| trans-epsilon viniferin | <LOQ | <LOQ | <LOD | <LOQ | <LOQ |
| Caffeine | 117.90 ± 5.89 | 218.91 ± 10.95 | 263.17 ± 13.16 | 198.83 ± 9.94 | 108.78 ± 5.44 |
| trans-polydatin | <LOQ | <LOQ | <LOQ | <LOQ | <LOD |
| ∑Others | 124.77 | 235.69 | 282.64 | 225.72 | 130.58 |
| Samples | O2•− | HOCl |
|---|---|---|
| IC50 (µg/mL) | ||
| S. ramosissima extract | 158.87 ± 11.96 a | 5.80 ± 0.40 a |
| Positive controls | ||
| Gallic acid | 23.82 ± 0.81 c | 0.61 ± 0.08 b |
| Catechin | 84.40 ± 4.58 b | 0.09 ± 0.01 b |
| Sample | Concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| 0.1 | 1 | 10 | 100 | 1000 | |
| S. ramosissimaextract | 97.39 ± 13.91 | 100.81 ± 14.86 | 86.15 ± 13.36 | 89.19 ± 18.59 | 86.11 ± 20.06 |
| Negative control | 0.00 ± 0.33 | ||||
| Positive control | 95.55 ± 9.09 | ||||
| Sample | Concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| 0.1 | 1 | 10 | 100 | 1000 | |
| S. ramosissimaextract | 99.41 ± 8.88 | 100.02 ± 5.00 | 101.27 ± 5.09 | 101.25 ± 4.66 | 107.05 ± 7.34 |
| Negative control | 0.00 ± 0.18 | ||||
| Positive control | 102.79 ± 9.54 | ||||
| Compounds Permeation (%) | Compounds Permeation (μg/mg dw) | |||||
|---|---|---|---|---|---|---|
| Time (H) | Rutin | Quercetin | Syringic Acid | Rutin | Quercetin | Syringic Acid |
| 0.5 | 3 | 7 | 2 | 0.017 ± 0.001 | 0.029 ± 0.001 | 0.015 ± 0.001 |
| 1 | 5 | 6 | 7 | 0.032 ± 0.002 | 0.026 ± 0.001 | 0.042 ± 0.02 |
| 2 | 6 | 10 | 9 | 0.041 ± 0.002 | 0.043 ± 0.002 | 0.056 ± 0.003 |
| 4 | 8 | 14 | 10 | 0.052 ± 0.003 | 0.058 ± 0.003 | 0.064 ± 0.003 |
| 8 | 7 | 21 | 8 | 0.047 ± 0.002 | 0.091 ± 0.005 | 0.052 ± 0.003 |
| 24 | 11 | 20 | 11 | 0.073 ± 0.004 | 0.087 ± 0.004 | 0.074 ± 0.004 |
| Epidermis | 1 | 5 | 3 | 0.007 ± 0.000 | 0.022 ± 0.001 | 0.020 ± 0.001 |
| Dermis | - | 3 | 3 | - | 0.012 ± 0.001 | 0.016 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.; Silva, A.M.; Moreira, M.M.; Salazar, M.; Švarc-Gajić, J.; Brezo-Borjan, T.; Cádiz-Gurrea, M.d.l.L.; Carretero, A.S.; Loschi, F.; Dall’Acqua, S.; et al. Salicornia ramosissima: A New Green Cosmetic Ingredient with Promising Skin Effects. Antioxidants 2022, 11, 2449. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11122449
Correia A, Silva AM, Moreira MM, Salazar M, Švarc-Gajić J, Brezo-Borjan T, Cádiz-Gurrea MdlL, Carretero AS, Loschi F, Dall’Acqua S, et al. Salicornia ramosissima: A New Green Cosmetic Ingredient with Promising Skin Effects. Antioxidants. 2022; 11(12):2449. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11122449
Chicago/Turabian StyleCorreia, Ana, Ana Margarida Silva, Manuela M. Moreira, Miguel Salazar, Jaroslava Švarc-Gajić, Tanja Brezo-Borjan, Maria de la Luz Cádiz-Gurrea, Antonio Segura Carretero, Francesca Loschi, Stefano Dall’Acqua, and et al. 2022. "Salicornia ramosissima: A New Green Cosmetic Ingredient with Promising Skin Effects" Antioxidants 11, no. 12: 2449. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11122449