1. Introduction
Chronic nephritis leads to an irreversible reduction in the glomerular filtration rate and renal fibrosis, ultimately leading to chronic kidney disease (CKD) and end-stage renal disease (ESRD). The infiltration of the glomerular and interstitial macrophages is a hallmark of CKD that plays a crucial role in renal injury [
1]. After kidney injury, cytokines or chemokines released by damaged cells recruit monocytes to inflammatory lesions, where they are activated and differentiated into macrophages [
2]. Macrophages are crucial immunological regulators and mediators of inflammation; therefore, the secretion of macrophage-derived inflammatory cytokines, such as interleukin (IL)-1 and IL-6, is known to induce kidney damage and inflammation [
3]. Inflammatory macrophages are activated and differentiated on-site, resulting in the release of IL-1, which induces the immune responses of the Th1-type cells that damage tissues [
4]. In CKD, immune cells infiltrating the kidneys play a deleterious role, actively participating in the disease’s progression and leading to nephron loss and fibrosis [
1,
5].
The NF-κB signaling pathway is highly activated in inflammatory diseases. It is accompanied by the recruitment of inflammatory cells and the production of proinflammatory cytokines, such as IL-1, IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α) [
6,
7]. The IκB kinase (IKK) complex, a vital component of the NF-κB signaling pathway, consists of two catalytically active kinases, IKKα and IKKβ, and the regulatory subunit IKKγ (NEMO). Notably, IKKβ reportedly induces the expression of cytokines through the phosphorylation of p65 of the NF-κB pathway [
8,
9]. In contrast, IKKα acts through canonical and non-canonical NF-κB pathways [
10]. Moreover, IKKα reportedly mediates macrophage polarization and the secretion of fibrotic factors [
11]. Several studies have examined the advantageous effect of suppressing IL-1 on the renal function [
12]. Interleukin-1β (IL-1β) is released primarily from stimulated macrophages and monocytes and plays a crucial role in inflammatory and immune responses [
13]. Notably, the IL-1β-dependent signaling drives renal damage and fibrosis by activating the proliferation of kidney stromal cells via the MYC transcription factor [
4,
14,
15]. Additionally, the infiltration of macrophages in the kidneys has been associated with the level of kidney injury and kidney fibrosis in human and experimental diabetic nephropathy [
5]. IL-1β contributes to renal damage; thus, its inhibition might offer a crucial way of preventing renal fibrosis.
Reactive oxygen species (ROS) are critical mediators of the activation of proinflammatory signaling pathways [
16]. Therefore, the production of ROS might favor the induction of proinflammatory macrophages during CKD progression. Elevation in the levels of ROS or reactive nitrogen species, or both, is commonly observed in animal models of CKD [
17,
18], similar to the progression of renal disease in humans [
19]. Moreover, increased intracellular levels of ROS cause the oxidation of proteins, lipids, and DNA, resulting in cellular damage in the kidneys. Hydrogen peroxide (H
2O
2), a major endogenous ROS, is a destructive molecule that is widely used to mimic ROS in in vitro studies [
20].
Heme oxygenase-1 (HO-1) is a cellular defense mechanism activated by oxidative stress and other stimuli. It also has antioxidant and anti-inflammatory properties. Unilateral ureteral obstruction caused more severe fibrosis in HO-1 knockout mice. Furthermore, macrophage infiltration was increased in HO-1-deficient mice, as measured by the expression of F4/80 [
21]. HO-1 is rapidly produced in the kidneys during glycerol-induced rhabdomyolysis, and previously, the stimulation of HO-1 with hemoglobin was shown to reduce injury [
22]. The preinduction of HO-1 increases renal function and survival, whereas the pharmacological suppression of HO-1 aggravates kidney disease. HO-1 suppresses the overactive immune response in several immune and kidney-resident cells [
23]. HO-1 regulates numerous pathways to mediate cytoprotection in kidney disease. Thus, the exploration of the mechanism of HO-1-mediated protection in the kidney after oxidative injury is crucial.
Sesamol (SM), 3,4-methylenedioxyphenol, is a lignan compound and an important aromatic component of sesame oil [
24]. SM is present in sesame (
Sesamum indicum) seeds and sesame oil and is continuously decomposed by sesaline and other substances during thermal processing. SM has a potent antioxidant capacity [
25], as well as anti-inflammatory, antiaging, and cardioprotective potential [
26,
27]. SM reduced the oxidative-stress-induced lipid peroxidation and malondialdehyde (MDA) levels of ApoE
–/– nephrectomy mice [
28].
In this study, we investigated the protective effects of SM on a 5/6 nephrectomized (5/6 Nx) mice model. In particular, we examined the influences of SM on macrophage infiltration and inflammatory mediators. Our study provides a novel mechanism of the action of SM in the prevention and treatment of renal fibrosis.
2. Materials and Methods
2.1. Cell Culture and Reagents
The Tohoku Hospital Pediatrics-1 (THP-1) cell line was purchased from the American Type Culture Collection (Rockville, MD, USA). THP-1 cells, a human leukemia monocytic cell line, were maintained in RPMI-1640 medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 25 U mL−1 penicillin and streptomycin and 10% fetal bovine serum (HyClone Laboratories, Logan, UT, USA). Sesamol SM (5-hydroxy-1,3-benzodioxole; purity: 98%), dimethyl sulfoxide (DMSO), phorbol 12-myristate 13-acetate (PMA), and H2O2 solution were purchased from Sigma (St. Louis, MO, USA). IKKα siRNA (si-IKKα) and control siRNA (si-Ctl) were procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Animal Design
All the mouse experiments were approved by the Institutional Animal Care and Use Committee of China Medical University and performed in accordance with the National Institutes of Health Laboratory Animal Care and Use Guidelines (NIH Publication No. 85-23, revised edition 1996). Female ApoE
–/– mice (Jackson Laboratory, Sacramento, CA, USA) aged 10–12 weeks (weighing 21–23 g) underwent 5/6 nephrectomy (5/6 Nx), the complete removal of the right kidney, and the infarction of approximately 2/3rd of the left kidney by ligating part of the renal artery branch. Female mice were used owing to their small glomerular sieving structure [
29], which is beneficial for the development of CKD. Moreover, lipid abnormalities often accompany and aggravate renal disease. ApoE
–/– mice, as a dyslipidemia animal model, that underwent 5/6Nx easily accumulated a greater number of immune cells in the kidneys, making them ideal for studying kidney inflammation. Throughout the study, the mice were fed on a regular diet. The mice received SM (25 or 50 mg/kg) via oral gavage thrice per week for eight weeks after 5/6 Nx surgery. All the mice were euthanized by isoflurane (Abbott Laboratories, Chicago, IL, USA) inhalation.
2.3. Plasma Biochemical Analyses
We separated the plasma from the whole blood and then removed the platelets from the plasma by centrifugation (centrifuge 5415 R, Eppendorf). The test paper and plasma (150 μL) were then placed in an automatic clinical chemistry analyzer (Arkray SPOTCHEM EZ SP-4430, Kyoto, Japan) according to the manufacturer’s instructions.
2.4. Histology Staining, Immunohistochemistry, and Masson’s Trichrome Staining
The kidney tissues were embedded in paraffin and sliced into 3 µm-thick sections. The slides were stained with hematoxylin and eosin (H&E, Abcam, Cambridge, UK), antibodies, or Masson’s trichrome stain (Sigma). Images were captured using a DM750 microscope (Leica, Wetzlar, Germany) and analyzed using the ImageJ software (version 1.52a, NIH, Bethesda, MD, USA).
2.5. Cell Viability Assay
For this assay, 1 × 104 THP-1 cells were inoculated on 96-well plates for the cell viability assessment. PMA was added for 48 h to render the cells adherent, after which the medium was changed to a fresh medium for 24 h. The cells were treated with H2O2 (0–30 µM) or SM (0–20 µM) for 24 h. Subsequently, the cells were incubated with 10% WST-1 reagent (Abcam) in a fresh medium at 37 °C for 4 h. The absorbance of each sample at 440 nm was measured using a microplate reader (Infinite M1000, TECAN, Mechelen, Belgium). The percentage of each sample was calculated after normalizing all the data to those of the corresponding controls.
2.6. Intracellular Reactive Oxidative Stress Measurement
The intracellular levels of ROS were determined using a cellular ROS/superoxide detection assay kit (Abcam) according to the manufacturer’s instructions. The cells (1 × 104) were inoculated on 96-well plates, and PMA was added to render cells adherent for 48 h, after which the medium was changed to fresh medium for 24 h. The THP-1 cells were treated with H2O2 (0–30 µM) for 24 h. Subsequently, the supernatant was removed, and the cells were washed with phosphate-buffered saline (PBS). The 2′,7′-dichlorofluorescein diacetate (DCFH-DA) ROS-specific stain was then added, and the cells were incubated in the dark at 37 °C for 30 min. The cells were then washed twice with PBS. The intracellular levels of ROS were determined using an Infinite M1000 microtiter plate reader (TECAN) (Ex = 488 nm, Em = 520 nm).
2.7. Immunoblot Analyses
THP-1 cells were seeded on six-well plates at a density of 1 × 107 cells per well and processed as previously described. The cells were then treated with vehicle, H2O2 (5 µM for 24 h) alone, or H2O2 after an SM (2 µM for 1 h) pretreatment. To obtain protein extracts, the cells were lysed and homogenized in RIPA lysis buffer in the presence of protease inhibitors (Roche Applied Science, Penzberg, Germany). After centrifugation, the protein concentration was determined using a BSA protein assay kit (Pierce Biotechnology Inc., Waltham, MA, USA). For the immunoblot assays, 20 µg of cell lysate was loaded onto 12% SDS-PAGE gel per well and separated by electrophoresis. The separated proteins were transferred to Hybond-PVDF membranes (GE Healthcare Amersham, Buckinghamshire, UK). The membranes were blocked with blocking buffer for 1 h at 25 °C, incubated overnight at 4 °C with primary antibodies (1:1000), and finally incubated with appropriate secondary antibodies (1:5000). The primary antibodies used included anti-p-IKKα (1:1000, #44-714) and anti-IKKα (1:1000, #14A231) (Invitrogen, Waltham, MA, USA), anti-p-NF-кB (1:1000, #3033) and anti-p-IкBα (1:1000, #9246) (Cell Signaling Technology, Danvers, MA, USA), anti-HO-1 (1:1000, ab13243, Abcam), and anti-β-actin (1:10,000, A5441, Sigma). β-actin was used as the loading control. The protein expression was evaluated using an ECL reagent (Millipore, Billerica, MA, USA). Images were obtained using quantitative video densitometry (G-box Image System; Syngene, Frederick, MD, USA).
2.8. Transfection of siRNA
The cells were inoculated on six-well plates at approximately 5 × 106 cells per well and stimulated with PMA for 48 h. The medium was replaced with fresh medium supplemented with 20% FBS without antibiotics. The control and IKKα-siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were prepared at a final concentration of 10 nM, following the manufacturer’s instructions. The control and IKKα-siRNA and the siRNA transfection reagent (INTERFERin®, Polyplus-transfection, Illkirch, France) were mixed for 10 min and then transfected into the cells for 12 h. H2O2 was then added to stimulate the cells.
2.9. Quantitative Real-Time PCR
THP-1 cells were seeded at a density of 1 × 10
6 cells/well on 6-well plates and treated with different stimuli. For the in vivo experiments, the murine renal tissues were homogenized. All the samples were collected and frozen with NucleoZOL (Macherey-Nagel, Düren, Germany) at −80 °C, and total RNA was isolated and extracted by centrifugation according to the manufacturer’s instructions. The iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) was used to synthesize the cDNA. Finally, the Q SYBR Green Supermix (Bio-Rad) was used for the real-time PCR. The primers used are listed in
Table 1.
2.10. Docking Pose Prediction
The crystal structures of IKKα (PDB code: 5EBZ, deposited date: 20 October 2015 and HO-1 (PDB code: 1N3U, deposited date: 29 October 2002) were obtained from the Protein Data Bank (PDB,
https://www.rcsb.org/) for the first step of the docking simulation. The docking of IKKα and HO-1 with the protein catalytic sites was evaluated using BIOVIA Discovery Studio (Dassault Systèmes, Vélizy-Villacoublay, France).
2.11. Statistical Analysis
The results are presented as the mean ± standard error of the mean (SEM) for each repeated experiment. Statistical significance (p < 0.05) was assessed by one-way ANOVA for multiple groups and Student’s t-test using the GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA).
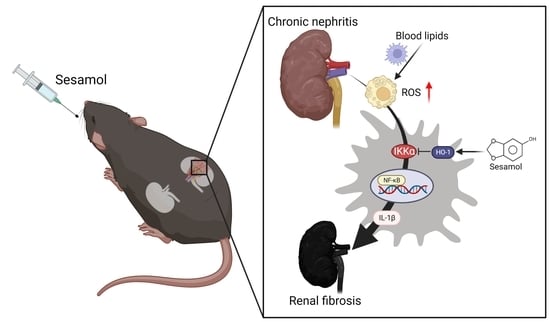
4. Discussion
Renal inflammation is an initial response to renal injury. Inflammation promotes the process of fibrosis, ultimately leading to CKD and ESRD. However, we lack effective strategies that can be used to improve this condition. SM is a natural organic compound extracted from sesame seeds with antioxidant and anti-inflammatory properties. Notably, 5/6 nephrectomy (5/6 Nx) in mice mimics renal failure after the loss of kidney function in humans, exacerbates renal injury through inflammation and oxidative stress, and has been widely used in CKD research [
28]. In this study, we demonstrated that SM ameliorated renal inflammation and fibrosis in 5/6 Nx ApoE
−/− mice. Moreover, we also observed that through the IKKα pathway, renal ROS induce the activation of macrophages and the secretion of IL-1β, which recruits more immune cells (nucleated cells, including macrophages) to the renal tissues, leading to the increased formation of foam cells and increased production of ROS, which cause inflammation and fibrosis in the kidneys, resulting in CKD. However, SM was shown to arrest this process via an increase in the levels of HO-1, which led to the inhibition of IKKα in the macrophages (
Figure 6).
Inflammation is closely related to renal disease and can be defined as a complex network of interactions between renal parenchymal cells and resident immune cells, including macrophages, dendritic cells, circulating monocytes, lymphocytes, and neutrophils. The kidney harbors various resident immune cells that play crucial roles in maintaining tissue homeostasis. These cells produce inflammatory mediators that initiate kidney disease and concomitantly trigger a regulatory response to curb inflammation, repair damaged tissue, and restore homeostasis. Inflammation plays a vital role in the progression of chronic renal failure. Excessive inflammation activates the white blood cells, leading to the production of cytokines related to splenomegaly. The inflammatory responses in each group of mice were compared by calculating the size of the spleen (data not shown). In this study, we observed that nucleated cells infiltrated the kidneys of the 5/6 Nx ApoE−/− mice, and this infiltration was ameliorated by SM.
Renal fibrosis is the final critical common pathway in the progression of renal diseases. Renal fibrosis has been associated with inflammatory cell infiltration, tubular atrophy, and the loss of peritubular or glomerular capillaries, during which a sustained inflammatory process might result in substantial tissue damage, eventually leading to fibrogenesis [
1,
12]. Therefore, anti-inflammatory therapies should be investigated for the treatment of renal fibrosis. Acute kidney injury (AKI) and CKD are associated with increased numbers and infiltration of the macrophages in the kidney [
1,
3]. Macrophages are a heterogeneous population of cells with diverse functions and phenotypic plasticity. They are also known to play pathogenic roles in renal inflammation and fibrosis. A recent study also supported the notion that macrophages directly contribute to the development of fibrosis by sustaining the renal inflammatory milieu [
5]. In addition, regardless of the etiology, renal parenchymal injury induces the release of chemotactic factors, renal infiltration of macrophages, and polarization to the M1 phenotype. M1 macrophages are characterized by the production of inflammatory cytokines, which can lead to tubular cell death, endothelial dysfunction, inflammation, and fibrosis, while suppressing M2 repolarization. Thus, the sustained infiltration of M1 macrophages might contribute to the progression of renal injury [
32]. The causal role of the classically activated macrophages in nephritis has been demonstrated by studies showing that macrophage depletion or the inhibition of monocyte chemoattractant proteins reduced the number of renal macrophages and protected the kidney [
5]. Inhibiting the infiltration of macrophages reduces proteinuria and structural damage to the kidney [
5,
12]. Thus, the inhibition of the macrophages is likely to be a beneficial approach for the treatment of CKD. In this study, we found that SM inhibited macrophage infiltration and the ROS-induced expression of IL-1β in the kidneys of ApoE
−/− 5/6 Nx mice.
CKD is associated with dyslipidemia, including high levels of triglycerides, low levels of HDL cholesterol, and an altered lipoprotein composition [
33]. Increasing evidence supports the notion that lipids are nephrotoxic [
29,
34]. The elevated levels of some lipids result in increased inflammation and oxidative stress in CKD. A high-fat diet causes lipid accumulation, as well as structural and functional abnormalities in the proximal tubular epithelial cells and the release of large amounts of proinflammatory cytokines [
35]. In our pilot study, we observed changes in the glomerular cluster structure and glomerular microvascular lipid accumulation, as indicated by the H&E staining and Oil Red O staining of the kidneys of the 5/6 Nx ApoE
−/− mice. This situation was improved after the oral administration of SM. Moreover, we assessed the levels of MDA to analyze the production and levels of ROS. The levels of ROS were increased in the ApoE
−/− mice with 5/6 Nx, which presented higher levels of triglycerides, total cholesterol, and LDL-c in the plasma and a higher inflammatory response in the kidneys. Regarding the reduction in glomerular lipid deposition in the 5/6 Nx mice after the SM treatment, we found that SM protected the tissue against ROS damage and improved glomerular damage attributed to ROS as the main inflammatory factor. Lipids promote the recruitment of immune cells, such as macrophages, neutrophils, and bone-marrow-derived dendritic cells, to the tissues. In patients with kidney disease, the inflammatory response was caused by an increase in the levels of triglyceride-rich lipoproteins, and patients on hemodialysis had significantly lower inflammation and levels of lipoprotein variables than patients with CKD [
36]. These findings implied that the beneficial effects of lowering the levels of triglyceride-rich lipoproteins might reduce the inflammatory response.
Macrophages, which are monocyte-derived phagocytic cells, are located in peripheral tissues and act as important mediators of inflammation and immune modulation [
2]. They are prevalent in the kidneys of humans with chronic renal disease. Classically activated macrophages release inflammatory cytokines and promote oxidative stress, leading to the development of renal fibrosis [
5,
32]. Although the macrophages play a crucial role in scavenging cellular debris, the increased renal infiltration of macrophages can tip the balance, thus causing local injury and inflammation. Injury and inflammation are mediated by the release of macrophage-derived inflammatory cytokines, such as interleukin (IL)-1, IL-6, and IL-23, and the generation of reactive oxygen/nitrogen species, each of which has been implicated in impaired renal function [
1,
3]. Generally, cytokines contribute to the pathogenesis of kidney disease by upregulating endothelial cell adhesion molecules and chemokines that further promote renal immune cell infiltration [
3]. In addition, many cytokine-activated signaling pathways increase the activation of IKK and NF-κB, further promoting a proinflammatory phenotype [
37]. Evidence suggesting that the NF-ĸB system is involved in the development of renal inflammation and injury has been described in studies of various clinical and experimental renal diseases. The expression or activation of NF-κB is increased in the kidneys of patients with glomerulonephritis, diabetic nephropathy, and AKI [
37]. One of the consequences of this inflammatory cycle is the promotion of local oxidative stress, which enhances renal injury and impairs the renal tubular and hemodynamic functions. In this study, we identified IKKα as a regulator of inflammation in kidney diseases. The IKK complex is essential for the activation of NF-кB during inflammation and oxidative stress stimulation. The NF-κB pathway consists of canonical and non-canonical pathways. The canonical NF-κB (p65/p50) pathway is activated by various stimuli, transducing a quick but transient transcriptional activity, to regulate the expression of various proinflammatory genes and serve as the critical mediator of the inflammatory response. The non-canonical NF-κB pathway is also an important arm of NF-κB signaling that predominantly targets the activation of the p52/RelB NF-κB complex. However, canonical NF-κB responses are much faster than non-canonical NF-κB pathway signaling, making this pathway particularly important for the innate immune response of first responder cells, such as the macrophages [
6,
7]. NF-кB is involved in the regulation of proinflammatory signals [
37]. The IKKβ subunit is a major activator of NF-кB and the underlying inflammatory response. Therefore, we used siIKKα to demonstrate that IKKα might induce the activation of NF-кB. Several studies support our findings [
38,
39]. Moreover, we observed that the stimulation of THP-1 cells with H
2O
2, which increased the expression of IL-1β, promoted inflammation. Conversely, this effect was inhibited by incubating the THP-1 cells with siIKKα or BMS345541 (an IKK inhibitor). Furthermore, considering that the inhibition of IKKα by siIKKα might affect the transcription and translation of IKKβ, we examined the protein expression of IKKβ and found that it was not affected. In addition, we also found that p-IKKα and the total IKKα were both elevated in the H
2O
2-induced cells. Another article also found similar results [
40]. These results indicated that H
2O
2 induced the expression of IL-1β in the THP-1 cells via the IKKα/NF-кB signaling.
As mentioned previously, research on the benefits of exploiting drug mechanisms in kidney disease is still lacking. In this study, we found that SM diminished the expression of IL-1β by inducing the expression of HO-1 and inhibiting the IKKα pathway. Furthermore, we confirmed the inhibitory effect of HO-1 on the IKK pathway by assessing the protein-binding site. In addition, although we did not explore fibrosis-related proteins, the findings of Masson’s trichrome staining were consistent with some studies reporting a synergistic role of IL-1β in renal fibrosis. Therefore, we concluded that sesame oil and nutritional supplements containing SM might be used to reduce the burden on the kidney function. In view of its safety, low price, and wide availability, SM can serve as a potential therapeutic agent for the treatment of kidney disease. However, more studies are required to determine the efficacy and safety of SM for patients with CKD.
This study had some limitations. Inflammation and lipids play significant roles in the progression of CKD. Macrophages take up lipids and readily form foam cells. The ApoE
−/− mice that underwent 5/6 Nx and did not receive the SM treatment accumulated a greater number of immune cells in the kidneys, indicating the formation and transportation of an increased number of foam cells in the blood, thus promoting glomerular lipid deposition. Foam cells are encountered in common glomerular diseases, such as focal and segmental glomerulosclerosis and diabetic nephropathy, but their pathogenic significance remains unknown. Extrapolating from studies on atherosclerosis, we can observe that therapeutics targeting the production of mitochondrial ROS or modulating cholesterol and lipoprotein uptake or their egress from these cells might prove beneficial for the treatment of kidney diseases in which foam cells are present. Secondly, different macrophages reflect different physiological roles in kidney disease. Thus, further understanding of the influences of specific subpopulations of macrophages will be helpful for the development of treatment strategies for kidney diseases. Furthermore, several studies have analyzed specific markers of each immune cell, which is a more accurate measurement method. However, this was another limitation of our study. Therefore, in future studies, the immune system should be thoroughly investigated to clarify the role of each component in kidney diseases. Thirdly, HO-2 is a factor affecting macrophage polarization and phagocytic activity in inflammation. Namely, HO-2 deficiency impairs HO-1 expression and inducibility in the macrophages [
41]. In other research, HO-2 deficiency was found to enhance STZ-induced renal dysfunction and morphological damage [
42]. However, HO-2 expression may reduce the effects of lipid peroxidation in the kidney [
43]. Thus, our future studies may focus on the expression of HO-2 and its underlying mechanism. Fourthly, although structural and functional problems affecting the kidney are the leading cause of CKD, the current therapeutic strategies target the blood pressure, blood sugar, and dyslipidemia, which do not address fundamental etiologies, such as inflammation and fibrosis. Although kidney transplantation is the best option for enhancing the kidney function, there are critical limitations affecting the identification of a suitable donor. Thus, developing novel therapeutic strategies for the treatment of CKD is crucial for addressing the structural problems and dysfunction of the damaged kidney. Sesamol, which is derived from sesame seeds, is a natural traditional health food that has been used in ancient medicine for a long time. Many studies point to the protective effect of sesamol on kidney disease. Therefore, SM is highly suitable as a treatment or nutritional supplement for CKD patients.