Effect of Debittering with Different Solvents and Ultrasound on Carotenoids, Tocopherols, and Phenolics of Lupinus albus Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
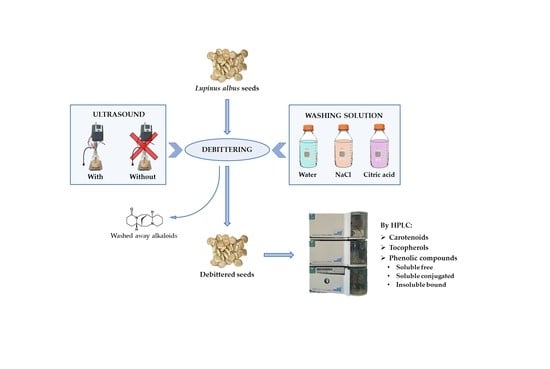
2.2.1. Debittering
- -
- Control method with water, according to Erbaş [28], with minor modifications [27], as follows: the seeds were hydrated (1:6 w/v seeds:water ratio) for 12 h at room temperature, then cooked in boiling water (hydrated seeds:water 1:3 w/v) for 1 h, replacing the water after 30 min, and rinsed with water (cooked seeds:water 1:3 w/v) for 5 days at room temperature, changing the water every 12 h.
- -
- Control method with 0.5% sodium chloride solution, as described by Villacrés et al. [31], as follows: The seeds were hydrated for 8 h at 80 °C (1:3 w/v seeds:0.5% NaCl solution ratio), then the liquid was changed and the seeds were cooked for 1 h at 91 °C (1:3 w/v), renewing the solvent after 30 min. Five washes were then carried out using 0.5% NaCl solution at 35 °C for up to 28 h (first and second wash: 1:15 w/v, 3 h each; third wash: 1:5 w/v, 16 h; fourth wash: 1:7.5 w/v, 3 h; fifth wash: 1:5 w/v, 3 h). Two final washes with water at 18 °C (seeds:water 1:5 w/v), the first lasting 18 h and the second 3 h, were carried out to eliminate the excess salinity.
- -
- Experimental method. A detailed description and a flowsheet of the proposed debittering approach is shown in Estivi et al. [25]. The following two different solutions were employed: 1% NaCl and 1% citric acid. Additionally, two different treatments were applied, without and with ultrasound, utilizing a UP400St ultrasonic homogenizer (Hielscher Ultrasonics GmbH, Teltow, Germany) operating at 24 kHz and mounting a 14 mm diameter probe to treat the seeds during washing (60% amplitude) and cooking (100% amplitude). After the last washing, 0 to 2 soakings with distilled water were carried out for 12 h to remove salt or citric acid from the seeds. For Lot 1, the final washing started at 25.5 h to interrupt the process after 28.5 h or 45 h. For Lot 2, the seeds were sampled at the end of the first (t1 = 45 h) and second (t2 = 57 h) final soaking.
- (1)
- The influence of sonication (with or without) and solvent (1% NaCl or 1% citric acid) on antioxidants. The seeds of Lot 1 were debittered following the experimental method proposed by Estivi et al. [25]. To achieve an identical and low residual alkaloid content, a soaking time of 45 h was applied for 1% NaCl and of 28.5 h for 1% citric acid solution. The control was debittered with water, according to Erbaş [28], with minor modifications [27].
- (2)
- The effect of solvent (1% NaCl or 1% citric acid) and total soaking time (45 or 57 h) on antioxidant content. The seeds of Lot 2 were debittered by the experimental method [25], but without sonication. These soaking times achieved very low residual alkaloid contents for the citric acid solution and for the NaCl solution, respectively [25]. The seeds that were debittered by the control method with water [27,28] and by the reference method with sodium chloride solution [31] provided the controls.
2.2.2. Moisture
2.2.3. Carotenoids and Tocopherols
2.2.4. Phenolic Compounds
2.3. Statistical Analysis
3. Results and Discussion
3.1. Effect of Sonication and Solvents on Antioxidant Compounds
3.1.1. Carotenoids and Tocopherols
3.1.2. Phenolic Compounds
3.2. Effect of Different Debittering Methods on Antioxidant Compounds
3.2.1. Carotenoids and Tocopherols
3.2.2. Phenolic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lampart-Szczapa, E.; Obuchowski, W.; Czaczyk, K.; Pastuszewska, B.; Buraczewska, L. Effect of lupine flour on the quality and oligosaccharides of pasta and crisps. Nahrung 1997, 41, 219–223. [Google Scholar] [CrossRef]
- Hall, R.S.; Johnson, S.K. Sensory acceptability of foods containing Australian sweet lupin (Lupinus angustifolius) flour. J. Food Sci. 2004, 69, 92–96. [Google Scholar] [CrossRef]
- Güemes-Vera, N.; Peña-Bautista, R.J.; Jiménez, M.C.; Dávila-Ortiz, G.; Calderón-Domínguez, G. Effective detoxification and decoloration of Lupinus mutabilis seed derivatives, and effect of these derivatives on bread quality and acceptance. J. Sci. Food Agric. 2008, 88, 1135–1143. [Google Scholar] [CrossRef]
- Jayasena, V.; Khu, W.S.; Nasar-Abbas, S.M. The development and sensory acceptability of lupin-based tofu. J. Food Qual. 2010, 33, 85–97. [Google Scholar] [CrossRef]
- Jayasena, V.; Leung, P.P.Y.; Nasar-Abbas, S.M. Effect of lupin flour substitution on the quality and sensory acceptability of instant noodles. J. Food Qual. 2010, 33, 709–727. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ipek, D.; Ghafoor, K.; Al Juhaimi, F.; Uslu, N.; Babiker, E.E.; Mohamed Ahmed, I.A.; Alsawmahi, O.N. Physico-chemical and sensory properties of chips produced using different lupin (Lupinus albus L.) flour formulations and cooking methods. Int. J. Food Sci. 2020, 56, 2780–2788. [Google Scholar] [CrossRef]
- Lo, B.; Kasapis, S.; Farahnaky, A. Lupin protein: Isolation and techno-functional properties, a review. Food Hydrocoll. 2021, 112, 106318. [Google Scholar] [CrossRef]
- Capraro, J.; Magni, C.; Fontanesi, M.; Budelli, A.; Duranti, M. Application of two-dimensional electrophoresis to industrial process analysis of proteins in lupin-based pasta. LWT 2008, 41, 1011–1017. [Google Scholar] [CrossRef]
- Duranti, M.; Consonni, A.; Magni, C.; Scarafoni, A.; Sessa, F. The major proteins of lupin seed: Characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Bez, J.; Petersen, I.L.; Joehnke, M.S.; Detzel, A.; Busch, M.; Krueger, M.; Ispiryan, L.; O’Mahony, J.A.; Arendt, E.K.; et al. Techno-functional, nutritional and environmental performance of protein isolates from blue lupin and white lupin. Foods 2020, 9, 230. [Google Scholar] [CrossRef]
- Santos-Sánchez, G.; Ponce-España, E.; López, J.C.; Álvarez-Sánchez, N.; Álvarez-López, A.I.; Pedroche, J.; Millán, F.; Millán-Linares, M.D.C.; Lardone, P.J.; Bejarano, I.; et al. A lupin (Lupinus angustifolius) protein hydrolysate exerts anxiolytic-like effects in Western diet-fed ApoE−/− mice. Int. J. Mol. Sci. 2022, 23, 9828. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, G.; Cruz-Chamorro, I.; Álvarez-Ríos, A.I.; Álvarez-Sánchez, N.; Rodríguez-Ortiz, B.; Álvarez-López, A.I.; Fernández-Pachón, M.-S.; Pedroche, J.; Millán, F.; Millán-Linares, M.D.C.; et al. Bioactive peptides from lupin (Lupinus angustifolius) prevent the early stages of atherosclerosis in Western diet-fed ApoE−/− mice. J. Agric. Food Chem. 2022, 70, 8243–8253. [Google Scholar] [CrossRef] [PubMed]
- Fadimu, G.J.; Farahnaky, A.; Gill, H.; Olalere, O.A.; Gan, C.Y.; Truong, T. In-silico analysis and antidiabetic effect of α-amylase and α-glucosidase inhibitory peptides from lupin protein hydrolysate: Enzyme-peptide interaction study using molecular docking approach. Foods 2022, 11, 3375. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Glorio-Paulet, P.; Estivi, L.; Locatelli, N.; Cordova-Ramos, J.S.; Hidalgo, A. Tocopherols, carotenoids and phenolics changes during Andean lupin (Lupinus mutabilis Sweet) seeds processing. J. Food Comp. Anal. 2022, 106, 104335. [Google Scholar] [CrossRef]
- Briceño Berru, L.B.; Glorio-Paulet, P.; Basso, C.; Scarafoni, A.; Camarena, F.; Hidalgo, A.; Brandolini, A. Chemical composition, tocopherol and carotenoid content of seeds from different Andean lupin (Lupinus mutabilis) ecotypes. Plant Foods Hum. Nutr. 2021, 76, 98–104. [Google Scholar] [CrossRef]
- Czubinski, J.; Grygier, A.; Siger, A. Lupinus mutabilis seed composition and its comparison with other lupin species. J. Food Comp. Anal. 2021, 99, 103875. [Google Scholar] [CrossRef]
- Estivi, L.; Grassi, S.; Briceño-Berrú, L.; Glorio-Paulet, P.; Camarena, F.; Hidalgo, A.; Brandolini, A. Free phenolic compounds, antioxidant capacity and FT-NIR survey of debittered Lupinus mutabilis seeds. Processes 2022, 10, 1637. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Von Baer, E.; Gross, R. Sweet strains of Lupinus mutabilis. Z. Pflanzenzucht. 1983, 91, 334–337. [Google Scholar]
- Reinhard, H.; Rupp, H.; Sager, F.; Streule, M.; Zoller, O. Quinolizidine alkaloids and phomopsins in lupin seeds and lupin containing food. J. Chromatogr. A 2006, 1112, 353–360. [Google Scholar] [CrossRef]
- Uauy, R.; Vivien-Gattas, R.V.; Yañez, E. Sweet lupins in human nutrition. World Rev. Nutr. Diet. 1995, 77, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Estivi, L.; Brandolini, A.; Condezo-Hoyos, L.; Hidalgo, A. Impact of low-frequency ultrasound technology on physical, chemical and technological properties of cereals and pseudocereals. Ultrason. Sonochem. 2022, 86, 106044. [Google Scholar] [CrossRef] [PubMed]
- Miano, A.C.; Rojas, M.L.; Augusto, P.E.D. Using ultrasound for improving hydration and debittering of Andean lupin grains. J. Food Process Eng. 2019, 42, e13170. [Google Scholar] [CrossRef]
- Estivi, L.; Buratti, S.; Fusi, D.; Benedetti, S.; Rodriguez, G.; Brandolini, A.; Hidalgo, A. Alkaloid content and taste profile assessed by electronic tongue of Lupinus albus seeds debittered by different methods. J. Food Comp. Anal. 2022, 114, 104810. [Google Scholar] [CrossRef]
- Carvajal-Larenas, F.E.; Linnemann, A.R.; Nout, M.J.R.; Koziol, M.; van Boekel, M.J.A.S. Lupinus mutabilis: Composition, uses, toxicology and debittering. Crit. Rev. Food Sci. Nutr. 2016, 56, 1454–1487. [Google Scholar] [CrossRef] [Green Version]
- Córdova-Ramos, J.S.; Glorio-Paulet, P.; Camarena, F.; Brandolini, A.; Hidalgo, A. Andean lupin (Lupinus mutabilis Sweet): Processing effects on chemical composition, heat damage and in vitro protein digestibility. Cereal Chem. 2020, 97, 827–835. [Google Scholar] [CrossRef]
- Erbaş, M. The effects of different debittering methods on the production of lupin bean snack from bitter Lupinus albus L. seeds. J. Food Qual. 2010, 33, 742–757. [Google Scholar] [CrossRef]
- Córdova-Ramos, J.S.; Glorio-Paulet, P.; Hidalgo, A.; Camarena, F. Effect of technological process on antioxidant capacity and total phenolic content of Andean lupine (Lupinus mutabilis Sweet). Sci. Agropec. 2020, 11, 157–165. [Google Scholar] [CrossRef]
- Villacrés, E.; Quelal, M.B.; Fernandez, E.; Garcìa, G.; Cueva, G.; Rosell, C.M. Impact of debittering and fermentation processes on the antinutritional and antioxidant compounds in Lupinus mutabilis Sweet. LWT 2020, 131, 109745. [Google Scholar] [CrossRef]
- Villacrés, E.; Álvarez, J.; Rossell, C. Effects of two debittering processes on the alkaloid content and quality characteristics of lupin (Lupinus mutabilis Sweet). J. Sci. Food Agric. 2020, 100, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Panfili, G.; Fratianni, A.; Irano, M. Normal-phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Hidalgo, A.; Gabriele, S.; Heun, M. Chemical composition of wild and feral diploid wheats and their bearing on domesticated wheats. J. Cereal Sci. 2015, 63, 122–127. [Google Scholar] [CrossRef]
- Varas Condori, M.; Pascual Chagman, G.; Barriga Sanchez, M.E.; Villegas Vilchez, L.F.; Ursetta, S.; Guevara, A.; Hidalgo, A. Effect of tomato (Solanum lycopersicum L.) lycopene-rich extract on the kinetics of rancidity and shelf-life of linseed (Linum usitatissimum L.) oil. Food Chem. 2020, 302, 125327. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Estivi, L.; Bertuglia, K.; Ivanova, N.; Jukić, M.; Komlenić, D.K.; Lukinac, J.; Hidalgo, A. Effect of tomato pomace addition on chemical, technological, nutritional, and sensorial properties of cream crackers. Antioxidants 2022, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Šaponjac, V.T.; Ćetković, G.; Šeregelj, V.; Čanadanović-Brunet, J.; Chiosa, D.; Brandolini, A. Antioxidant properties and heat damage of water biscuits enriched with sprouted wheat and barley. LWT 2019, 114, 108423. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I.; Fernández, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Magalhães, S.C.Q.; Taveira, M.; Cabrita, A.R.; Fonseca, A.J.; Valentão, P.; Andrade, P.B. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Stamatovska, V.; Dimov, I. Effect of grape pomace powder addition on chemical, nutritional and technological properties of cakes. LWT 2020, 134, 109950. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Milla, R.; Martín-Robles, N.; Arc, E.; Kranner, I.; Becerril, J.M.; García-Plazaola, J.I. Side-effects of domestication: Cultivated legume seeds contain similar tocopherols and fatty acids but less carotenoids than their wild counterparts. BMC Plant Biol. 2014, 14, 1599. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Errington, S.; Yap, H.H. Studies on carotenoids from lupin seeds. In Proceedings of the 12th International Lupin Conference “Lupins for Health and Wealth”, Fremantle, Western Australia, 14–18 September 2008; Palta, J.A., Berger, J.B., Eds.; International Lupin Association: Canterbury, New Zealand, 2008. [Google Scholar]
- El-Difrawi, E.A.; Hudson, B.J.F. Identification and estimation of carotenoids in the seeds of four Lupinus species. J. Sci. Food Agric. 1979, 30, 1168–1170. [Google Scholar] [CrossRef]
- Boschin, G.; Arnoldi, A. Legumes are valuable sources of tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P.; Manunza, P.; Arnoldi, A.; Boschin, G. Quality of Lupinus albus L. (white lupin) seed: Extent of genotypic and environmental effects. J. Agric. Food Chem. 2014, 62, 6539–6545. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Frías, J.; Vidal-Valverde, C. Functional lupin seeds (Lupinus albus L. and Lupinus luteus L.) after extraction of α-galactosides. Food Chem. 2006, 98, 291–299. [Google Scholar] [CrossRef]
- Lampart-Szczapa, E.; Korczak, J.; Nogala-Kalucka, M.; Zawirska-Wojtasiak, R. Antioxidant properties of lupin seed products. Food Chem. 2003, 83, 279–285. [Google Scholar] [CrossRef]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Sun, T.; Tadmor, Y.; Li, L. Pathways for carotenoid biosynthesis, degradation, and storage. In Plant and Food Carotenoids. Methods in Molecular Biology; Rodríguez-Concepción, M., Welsch, R., Eds.; Humana: New York, NY, USA, 2020; Volume 2083, pp. 3–23. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Kentish, D.S.; Mawson, R.; Simons, L.; Vilkhu, K.; Versteeg, C.K. Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov. Food Sci. Emerg. Technol. 2008, 9, 155–160. [Google Scholar] [CrossRef]
- Feng, H.; Yang, W. Ultrasound processing. In Nonthermal Processing Technologies for Food; Zhang, H.Q., Barbosa-Cánovas, G.V., Balasubramaniam, V.M., Dunne, C.P., Farkas, D.F., Yuan, J.T.C., Eds.; John Wiley & Sons, Wiley-Blackwell/IFT Press: New Delhi, India, 2011; pp. 135–154. [Google Scholar]
- Kim, H.J.; Min, D.B. Chemistry of lipid oxidation. In Food Lipids: Chemistry, Nutrition, and Biotechnology, 3rd ed.; Akoh, C.C., Min, D.B., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 299–320. [Google Scholar]
- Karamać, M.; Orak, H.H.; Amarowicz, R.; Orak, A.; Piekoszewski, W. Phenolic contents and antioxidant capacities of wild and cultivated white lupin (Lupinus albus L.) seeds. Food Chem. 2018, 258, 1–7. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Compos. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]

| Control H2O | 1% NaCl | 1% NaCl US | 1% Citric Acid | 1% Citric Acid US | |
|---|---|---|---|---|---|
| (0.98 ± 0.11 g alkaloids/kg DM) | (1.02 ± 0.07 g alkaloids/kg DM) | ||||
| Carotenoids | |||||
| (α + β)-carotene | 2.25 ab ± 0.36 | 2.44 ab ± 0.09 | 2.41 ab ± 0.05 | 2.49 a ± 0.07 | 2.02 b ± 0.02 |
| β-cryptoxanthin | 0.18 c ± 0.02 | 0.11 d ± 0.00 | 0.10 d ± 0.00 | 0.42 b ± 0.00 | 0.50 a ± 0.03 |
| Lutein | 7.91 ab ± 0.86 | 8.74 a ± 0.47 | 8.35 a ± 0.03 | 8.05 a ± 0.27 | 6.83 b ± 0.03 |
| Zeaxanthin | 1.86 ab ± 0.14 | 1.95 a ± 0.10 | 1.94 ab ± 0.01 | 1.74 b ± 0.05 | 1.50 c ± 0.01 |
| Total carotenoids | 12.20 ab ± 1.38 | 13.23 a ± 0.66 | 12.80 a ± 0.01 | 12.70 a ± 0.39 | 10.85 b ± 0.08 |
| Tocopherols | |||||
| α-tocopherol | 0.45 b ± 0.10 | 0.46 ab ± 0.04 | 0.56 a ± 0.03 | 0.50 ab ± 0.01 | 0.44 b ± 0.00 |
| β-tocopherol | 1.36 b ± 0.51 | 1.01 b ± 0.17 | 0.85 b ± 0.25 | 2.64 a ± 0.02 | 2.09 a ± 0.15 |
| γ-tocopherol | 191.58 ± 27.96 | 188.73 ± 17.08 | 175.82 ± 0.06 | 182.93 ± 3.22 | 169.04 ± 3.38 |
| δ-tocopherol | 1.99 ± 0.40 | 1.77 ± 0.06 | 1.61 ± 0.12 | 1.56 ± 0.52 | 1.24 ± 0.08 |
| Total tocopherols | 195.38 ± 28.97 | 191.97 ± 16.92 | 178.83 ± 0.47 | 187.63 ± 3.75 | 172.82 ± 3.32 |
| Control H2O | 1% NaCl | 1% NaCl US | 1% Citric Acid | 1% Citric Acid US | |
|---|---|---|---|---|---|
| (0.98 ± 0.11 g alkaloids/kg DM) | (1.02 ± 0.07 g alkaloids/kg DM) | ||||
| Soluble-free phenolics | |||||
| Genistein der. | 20.88 e ± 0.46 | 66.12 c ± 0.03 | 54.33 d ± 1.21 | 103.21 b ± 0.27 | 117.92 a ± 1.94 |
| Sinapic acid der. | 120.60 a ± 0.18 | 107.49 a ± 0.48 | 89.30 b ± 1.57 | 85.91 b ± 7.16 | 66.40 c ± 12.07 |
| Naringenin der. | nd | nd | nd | 4.11 a ± 0.14 | 3.58 b ± 0.01 |
| Diosmin der. | nd | nd | nd | 3.94 a ± 0.06 | 3.24 b ± 0.00 |
| Apigenin der. | 30.53 e ± 0.08 | 101.90 c ± 1.08 | 92.08 d ± 1.97 | 163.96 a ± 3.38 | 134.99 b ± 0.88 |
| Total free | 172.02 e ± 0.21 | 275.51 c ± 1.59 | 235.70 d ± 1.61 | 361.13 a ± 4.25 | 326.12 b ± 11.00 |
| Soluble-conjugated phenolics | |||||
| Genistein der. | 33.63 c ± 2.58 | 82.80 b ± 10.78 | 97.76 ab ± 13.13 | 111.76 a ± 4.82 | 99.15 ab ± 1.71 |
| Genistein | nd | nd | nd | 1.55 a ± 0.06 | 0.44 b ± 0.05 |
| Naringenin der. | 5.46 a ± 0.39 | 1.97b ± 0.73 | 1.83 b ± 0.45 | 2.08 b ± 0.20 | 1.25 b ± 0.03 |
| Catechin der. | 0.41 c ± 0.04 | nd | nd | 3.54 a ± 0.11 | 2.53 b ± 0.05 |
| Apigenin der. | 39.49 a ± 3.02 | 0.52 b ± 0.10 | 0.54 b ± 0.02 | nd | nd |
| Total conjugated | 78.99 c ± 6.03 | 85.28 bc ± 11.6 | 100.13 abc ± 12.7 | 118.94a ± 5.06 | 103.37 ab ± 1.68 |
| Insoluble-bound phenolics | |||||
| Genistein der. | 40.41 b ± 6.07 | 53.71 a ± 4.37 | 52.50 a ± 3.45 | 29.23 b ± 4.23 | 34.76 b ± 3.64 |
| Naringenin der. | 14.27 ± 0.57 | 18.39 ± 3.39 | 19.02 ± 1.15 | 15.25 ± 1.69 | 14.63 ± 1.04 |
| Catechin der. | 26.24 a ± 0.53 | 28.25 a ± 0.98 | 26.5 a ± 2.06 | 8.74 b ± 0.95 | 8.38 b ± 0.97 |
| Apigenin der. | 1.77 b ± 0.13 | 0.84 b ± 0.22 | 0.90 b ± 0.09 | 6.01 a ± 0.82 | 5.91 a ± 0.12 |
| Total bound | 82.69 b ± 7.30 | 101.19 a ± 8.53 | 98.92 ab ± 6.57 | 59.23 c ± 7.69 | 63.68 c ± 3.83 |
| Total phenolics | 333.69 d ± 13.13 | 461.98 bc ± 4.66 | 434.75 c ± 17.66 | 539.29 a ± 6.88 | 493.17 b ± 16.51 |
| Control H2O | Control 0.5% NaCl | 1% NaCl 45 h | 1% NaCl 57 h | 1% Citric Acid 45 h | 1% Citric Acid 57 h | |
|---|---|---|---|---|---|---|
| Carotenoids | ||||||
| (α + β)-carotene | 7.86 c ± 0.23 | 5.30 e ± 0.22 | 7.10 d ± 0.04 | 6.89 d ± 0.03 | 12.01 a ± 0.15 | 9.63 b ± 0.06 |
| β-cryptoxanthin | 1.58 c ± 0.04 | 1.12 d ± 0.19 | 1.29 d ± 0.01 | 1.21 d ± 0.03 | 3.05 a ± 0.05 | 2.60 b ± 0.06 |
| Lutein | 9.33 a ± 0.09 | 8.06 b ± 0.04 | 9.17 a ± 0.19 | 9.35 a ± 0.06 | 7.97 b ± 0.02 | 7.70 c ± 0.05 |
| Zeaxanthin | 2.30 a ± 0.06 | 1.90 c ± 0.04 | 2.07 b ± 0.03 | 1.89 c ± 0.01 | 2.06 b ± 0.03 | 1.85 c ± 0.03 |
| Tocopherols | ||||||
| α-tocopherol | 0.26 b ± 0.08 | 0.47 a ± 0.03 | 0.51a ± 0.01 | 0.50 a ± 0.00 | 0.29 b ± 0.02 | 0.31 b ± 0.02 |
| β-tocopherol | 1.13 ab ± 0.10 | 1.30 a ± 0.16 | 0.56d ± 0.00 | 0.52 d ± 0.01 | 0.99 bc ± 0.02 | 0.99 c ± 0.07 |
| γ-tocopherol | 237.77 a ± 4.34 | 197.33 cd ± 3.07 | 230.53a ± 2.66 | 214.27 b ± 2.65 | 208.35 bc ± 6.91 | 196.79 d ± 6.33 |
| δ-tocopherol | 2.09 bc ± 0.02 | 1.98 cd ± 0.01 | 1.82d ± 0.08 | 2.26 b ± 0.03 | 2.45 a ± 0.09 | 2.23 b ± 0.15 |
| Control H2O | Control 0.5% NaCl | 1% NaCl 45 h | 1% NaCl 57 h | 1% Citric Acid 45 h | 1% Citric Acid 57 h | |
|---|---|---|---|---|---|---|
| Soluble-free phenolics | ||||||
| Sinapic acid der. | 53.14 c ± 0.33 | 50.43 c ± 0.48 | 134.15 a ± 4.20 | 82.60 b ± 1.86 | 32.10 d ± 1.56 | 12.76 e ± 0.34 |
| Naringenin der. | 2.58 a ± 0.04 | 2.27 b ± 0.03 | 2.37 b ± 0.01 | 2.27 b ± 0.03 | 1.73 c ± 0.06 | 1.16 d ± 0.05 |
| Diosmin der. | 1.31 f ± 0.04 | 1.63 e ± 0.04 | 3.62 b ± 0.05 | 2.76 c ± 0.05 | 4.81 a ± 0.07 | 2.43 d ± 0.05 |
| Apigenin der. | 49.83 e ± 1.41 | 96.66 d ± 1.67 | 163.25 b ± 2.72 | 128.73 c ± 1.68 | 207.93 a ± 2.26 | 165.97 b ± 2.79 |
| Soluble-conjugated phenolics | ||||||
| Genistein der. | 73.04 c ± 2.59 | 65.63 d ± 1.14 | 57.01 e ± 2.01 | 63.22 d ± 2.86 | 105.62 a ± 1.60 | 81.18 b ± 1.60 |
| Genistein | 2.68 e ± 0.02 | 3.71 c ± 0.02 | 2.56 f ± 0.03 | 3.15 d ± 0.03 | 4.17 a ± 0.02 | 3.97 b ± 0.03 |
| Naringenin der. | 6.36 a ± 0.09 | 2.68 e ± 0.05 | 2.80 de ± 0.13 | 3.01 d ± 0.19 | 4.87 b ± 0.11 | 3.61 c ± 0.06 |
| Catechin der. | nd | 2.22 b ± 0.06 | 1.54 d ± 0.03 | 0.88 e ± 0.03 | 4.06 a ± 0.05 | 1.98 c ± 0.07 |
| Insoluble-bound phenolics | ||||||
| Genistein der. | 78.23 c ± 2.76 | 94.50 b ± 2.87 | 112.91 a ± 1.43 | 83.44 c ± 5.57 | 83.91 c ± 4.85 | 93.91 b ± 4.19 |
| Genistein | 2.13 a ± 0.02 | 1.06 d ± 0.03 | 1.51 c ± 0.09 | 1.75 b ± 0.09 | 1.77 b ± 0.04 | 1.52 c ± 0.01 |
| Naringenin der. | 10.89 c ± 0.91 | 13.24 ab ± 1.01 | 14.42 a ± 1.48 | 11.42 bc ± 0.84 | 11.44 bc ± 0.22 | 11.66 bc ± 0.15 |
| Catechin der. | 23.94 b ± 0.91 | 28.57 a ± 0.97 | 27.39 a ± 0.13 | 27.37 a ± 0.05 | 11.70 c ± 0.06 | 11.63 c ± 0.06 |
| Apigenin der. | 0.15 d ± 0.04 | 0.30 c ± 0.03 | 0.49 b ± 0.06 | 0.42 b ± 0.01 | 1.35 a ± 0.00 | 1.27 a ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estivi, L.; Fusi, D.; Brandolini, A.; Hidalgo, A. Effect of Debittering with Different Solvents and Ultrasound on Carotenoids, Tocopherols, and Phenolics of Lupinus albus Seeds. Antioxidants 2022, 11, 2481. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11122481
Estivi L, Fusi D, Brandolini A, Hidalgo A. Effect of Debittering with Different Solvents and Ultrasound on Carotenoids, Tocopherols, and Phenolics of Lupinus albus Seeds. Antioxidants. 2022; 11(12):2481. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11122481
Chicago/Turabian StyleEstivi, Lorenzo, Davide Fusi, Andrea Brandolini, and Alyssa Hidalgo. 2022. "Effect of Debittering with Different Solvents and Ultrasound on Carotenoids, Tocopherols, and Phenolics of Lupinus albus Seeds" Antioxidants 11, no. 12: 2481. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11122481