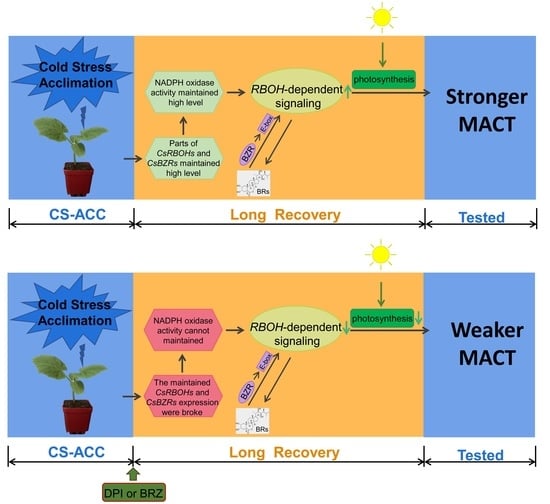
Photosynthesis Mediated by RBOH-Dependent Signaling Is Essential for Cold Stress Memory
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Materials and Temperature Treatments
- (1)
- CK: 25 °C.
- (2)
- Cold stress induction (CS-I) combined with a short recovery: 10 (1 h) and 25 °C (1.5 h).
- (3)
- Cold stress induction (CS-I) combined with a long recovery: 10 (1 h) and 25 °C (48 h).
- (4)
- Cold stress acclimation (CS-ACC) combined with a long recovery: 10 (1 h), 25 (1.5 h), 1 (3 h), and 25 °C (48 h).
- (5)
- DPI was sprayed on the seedlings after CS-ACC (CS-ACC-DPI) combined with a long recovery: 10 (1 h), 25 (1.5 h), and 1 °C (3 h), 50 mmol·L−1 DPI, 25 °C (48 h).
- (6)
- BRZ was sprayed onto the seedlings after CS-ACC (CS-ACC-BRZ) combined with a long recovery: 10 (1 h), 25 (1.5 h), and 1 °C (3 h), 50 mmol·L−1 BRZ, 25 °C (48 h).
- Grade 0: cotyledons and true leaves were intact without obvious injury symptoms.
- Grade 1: green cotyledons were wilted and the leaf margins on true leaves were wilted and curly.
- Grade 2: dehydration spots were apparent on the true leaves but were less than 1/2 of the total leaf area.
- Grade 3: the area of dehydration spots on the true leaves was 1/2 of the leaf area and the heart leaf margin was damaged.
- Grade 4: the area of dehydration spots on true and heart leaves was more than 1/2 of the leaf area.
- Grade 5: the whole plant was dehydrated and wilted.
2.2. Genome-Wide Identification of the RBOH and BZR Families
2.3. Phylogenetic Tree, Gene Structure, and Conserved Domains in RBOH and BZR Families
2.4. Cis-Acting Element Analysis
2.5. RNA Extraction and qRT-PCR Analysis
2.6. Chlorophyll Fluorescence-Induced Kinetic Curve (OJIP) Measurement
2.7. Measurement of the Electrolytic Leakage; Contents of Proline, H2O2, and Chlorophyll; and NADPH Oxidase Activity
2.8. Statistical Analysis
3. Results
3.1. Cold Stress Induced the Acquisition of Cold Stress Memory in Cucumber Seedlings and Enhanced the Acquired Cold Tolerance
3.2. Genome-Wide Analysis of the RBOH Protein Family in Cucumber
3.3. Collinearity Analysis of the RBOH Family in Different Plant Species
3.4. A Large Number of BZR-Binding Sites Exist in the Promoter of the RBOH Family
3.5. Inhibition of BRs or RBOH-Dependent Signaling Affected the Maintenance of the Expression of Four CsRBOHs during Recovery after CS-ACC
3.6. NADPH Oxidase Encoded by CsRBOHs Showed High Levels during Recovery after CS-ACC and Is Essential for Cold Stress Memory
3.7. Photosynthesis Efficiency Mediated by CsRBOH-Dependent Signaling Was Essential for Cold Stress Memory
3.8. Genome-Wide Analysis of the BZR Family in Cucumber
3.9. Inhibition of BRs or RBOH-Dependent Signaling Affected the Maintenance of the Expression of Two CsBZRs during Recovery after CS-ACC
3.10. BRs Are Involved in the Cold Stress Memory of Cucumber Seedlings
4. Discussion
4.1. Maintenance of Acquired Cold Tolerance Occurs in Cucumber Seedlings
4.2. Both BRs and RBOH-Dependent Signaling Are Essential for Cold Stress Memory in Cucumber Seedlings
4.3. The Repair of Photosynthetic Efficiency Mediated by CsRBOHs-Dependent Signaling during Recovery after CS-ACC Is Important for Cold Stress Memory in Cucumber
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; He, Y.H. Experiencing winter for spring flowering: A molecular epigenetic perspective on vernalization. J. Integr. Plant Biol. 2020, 62, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Faivre, L.; Bäurle, I.; Schubert, D. Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ. 2018, 42, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Kaplinsky, N.J.; Hu, C.; Charng, Y.Y. Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci. 2012, 195, 10–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, Z.; Browse, J. Cold comfort farm: The acclimation of plants to freezing temperatures. Plant Cell Environ. 2000, 23, 893–902. [Google Scholar] [CrossRef]
- Luo, W.; Huan, Q.; Xu, Y.Y.; Qian, W.F.; Chong, K.; Zhang, J.Y. Integrated global analysis reveals a vitamin E-vitamin K1 sub-network, downstream of COLD1, underlying rice chilling tolerance divergence. Cell Rep. 2021, 36, 109397. [Google Scholar] [CrossRef]
- Guo, A.Y.; Zhu, Q.H.; Chen, X.; Luo, J.C. GSDS: A gene structure display server. Hereditas 2007, 29, 1023–1026. [Google Scholar] [CrossRef]
- Chang, J.; Guo, Y.; Li, J.; Su, Z.; Wang, C.; Zhang, R.; Wei, C.; Ma, J.; Zhang, X.; Li, H. Positive Interaction between H2O2 and Ca2+ Mediates Melatonin-Induced CBF Pathway and Cold Tolerance in Watermelon (Citrullus lanatus L.). Antioxidants 2021, 10, 1457. [Google Scholar] [CrossRef]
- Eom, S.H.; Ahn, M.-A.; Kim, E.; Kim, S.K.; Lee, H.J.; Lee, J.H.; Hwanwi, S.; Lim, H.B.; Hyun, T.K. Plant Response to Cold Stress: Cold Stress Changes Antioxidant Metabolism in Heading Type Kimchi Cabbage (Brassica rapa L. ssp. Pekinensis). Antioxidants 2022, 11, 700. [Google Scholar] [CrossRef]
- Kim, J.M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef] [Green Version]
- Avramova, Z. Transcriptional ‘memory’ of a stress: Transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 2015, 83, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zuther, E.; Schaarschmidt, S.; Fischer, A.; Erban, A.; Pagter, M.; Mubeen, U.; Giavalisco, P.; Kopka, J.; Sprenger, H.; Hincha, D.K. Molecular signatures of increased freezing tolerance due to low temperature memory in Arabidopsis. Plant Cell Environ. 2018, 42, 854–873. [Google Scholar] [CrossRef] [PubMed]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.Y.; Ko, S.S.; Yeh, K.C.; Charng, Y.Y. Isolation and characterization of tomato Hsa32 encoding a novel heat-shock protein. Plant Sci. 2006, 170, 976–985. [Google Scholar] [CrossRef]
- Meiri, D.; Breiman, A. Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J. 2009, 59, 387–399. [Google Scholar] [CrossRef]
- Finka, A.; Cuendet, A.F.H.; Maathuis, F.J.M.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Ding, Y.; Shi, Y.; Ma, L.; Wang, Y.; Song, C.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef]
- Liu, J.; Whalley, H.J.; Knight, M.R. Combining modelling and experimental approaches to explain how calcium signatures are decoded by calmodulin-binding transcription activators (CAMTAs) to produce specific gene expression responses. New Phytol. 2015, 208, 174–187. [Google Scholar] [CrossRef] [Green Version]
- Kidokoro, S.; Yoneda, K.; Takasaki, H.; Takahashi, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Different cold-signaling pathways function in the responses to rapid and gradual decreases in temperature. Plant Cell 2017, 29, 760–774. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.-C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.A.; et al. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell 2017, 43, 618–629.e615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.S.N.; Poovaiah, B.W. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013, 75, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, H.; Zhang, X.; Xie, Q.; Gong, Z.; Yang, S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 2015, 32, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant mitogen-activated protein kinase cascades in environmental stresses. Int. J. Mol. Sci. 2021, 22, 1543. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.X.; Yu, J.Q. RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J. Exp. Bot. 2014, 65, 595–607. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.P.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2021, 18. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, D.G.J.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Yoshioka, H.; Numata, N.; Nakajima, K.; Katou, S.; Kawakita, K.; Rowland, O.; Jones, J.D.G.; Doke, N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to phytophthora infestans. Plant Cell 2003, 15, 706–718. [Google Scholar] [CrossRef] [Green Version]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Burian, M.; Podgórska, A.; Ostaszewska-Bugajska, M.; Szal, B.Z. Respiratory Burst Oxidase Homolog D as a Modulating Component of Oxidative Response under Ammonium Toxicity. Antioxidants 2022, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; Jiang, F.L.; Cen, B.J.; Huo, H.Q.; Wu, Z. Antioxidant enzymes act as indicators predicting intension of acquired and maintenance of acquired thermotolerance and the relationships between basal, acquired and maintenance of acquired thermotolerance of tomato. Sci. Hortic. 2019, 247, 130–137. [Google Scholar] [CrossRef]
- Sun, M.T.; Jiang, F.L.; Cen, B.J.; Wen, J.Q.; Zhou, Y.Z.; Wu, Z. Respiratory burst oxidase homologue-dependent H2O2 and chloroplast H2O2 are essential for the maintenance of acquired thermotolerance during recovery after acclimation. Plant Cell Environ. 2018, 41, 2373–2389. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and Type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate regulates the inducer of CBF expression-c-repeat binding factor/DRE binding factor1 Cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ye, K.Y.; Shi, Y.T.; Cheng, J.K.; Zhang, X.Y.; Yang, S.H. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.X.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, E5982–E5991. [Google Scholar] [CrossRef] [Green Version]
- CañODelgado, A.; Yin, Y.H.; Yu, C.; Vafeados, D.; Mora-Garcí, A.S.; Cheng, J.C.; Nam, K.H.; Li, J.M.; Chory, J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 2004, 131, 5341–5351. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.J.; Zhou, Y.H.; Ding, J.; Shi, K.; Asami, T.; Chen, Z.X.; Yu, J.Q. Induction of systemic stress tolerance by brassinosteroid in cucumis sativus. New Phytol. 2011, 191, 706–720. [Google Scholar] [CrossRef]
- Guo, H.Q.; Li, L.; Aluru, M.; Aluru, S.; Yin, Y.H. Mechanisms and networks for brassinosteroid regulated gene expression. Curr Opin. Plant Biol. 2013, 16, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Belkhadir, Y.; Jaillais, Y. The molecular circuitry of brassinosteroid signaling. New Phytol. 2015, 206, 522–540. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.X.; Yu, J.Q. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.; Nagy, L.; Hein, R.; Rascher, U.; Beierkuhnlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 2011, 71, 34–40. [Google Scholar] [CrossRef]
- Martin, A.P.; Michelazzo, C.; Ruiz, J.M.T.; Flexas, J.; Fernández, J.E.; Sebastiani, L.; Espejo, A.D. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins. J. Exp. Bot. 2014, 65, 3143–3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galle, A.; Sarasa, I.F.; Tomas, M.; Pou, A.; Medrano, H.; Carbo, M.R.; Flexas, J. The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): Acclimation or limitation? J. Exp. Bot. 2009, 60, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.T.Ö.; Kayihan, C.; Eyidogan, F.; Ekmekci, Y.; Yucel, M.; Öktem, H.A. Evaluation of photosynthetic performance of wheat cultivars exposed to boron toxicity by the JIP fluorescence test. Photosynthetica 2014, 52, 555–563. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef] [Green Version]
- Barrero-Gil, J.; Huertas, R.; Rambla, J.L.; Granell, A.; Salinas, J. Tomato plants increase their tolerance to low temperature in a chilling acclimation process entailing comprehensive transcriptional and metabolic adjustments. Plant Cell Environ. 2016, 39, 2303–2318. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Račková, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Janeeshma, E.; Kalaji, H.M.; Puthur, J.T. Differential responses in the photosynthetic efficiency of Oryza sativa and Zea mays on exposure to Cd and Zn toxicity. Acta Physiol. Plant 2021, 43, 12. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.Z.; Shah, J.; Schlauch, K.; et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.F.; Chen, J.C.; Zhang, X.L. Genetic regulation of shoot architecture in cucumber. Hortic. Res. 2021, 8, 143. [Google Scholar] [CrossRef]
- Dong, S.Y.; Wang, W.P.; Bo, K.L.; Miao, H.; Song, Z.C.; Wei, S.; Zhang, S.P.; Gu, X.F. Quantitative trait loci mapping and candidate gene analysis of low temperature tolerance in cucumber seedlings. Front. Plant Sci. 2019, 10, 1620. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Liu, W.Q.; Zhang, R.Y.; Xiang, C.G.; Zhang, R.Y.; Wang, Q.; Wang, T.; Li, X.J.; Lu, X.H.; Gao, S.L.; Liu, Z.X.; et al. Transcriptomic and physiological analysis reveal that α-Linolenic acid biosynthesis responds to early chilling tolerance in pumpkin rootstock varieties. Front. Plant Sci. 2021, 12, 685. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using RealTime Quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Di, Q.H.; Li, J.; Du, Y.F.; Wei, M.; Shi, Q.H.; Li, Y.; Yang, F.J. Combination of red and blue lights improved the growth and development of eggplant (Solanum melongena L.) seedlings by regulating photosynthesis. J. Plant Growth Regul. 2020, 40, 1477–1492. [Google Scholar] [CrossRef]
- Strasserf, R.J.; Srivastava, A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Masojídek, J.; Vonshak, A.; Torzillo, G. Chlorophyll fluorescence applications in microalgal mass cultures. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications. Developments in Applied Phycology; Sugget, D., Prášil, O., Borowitzka, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 4, pp. 277–292. [Google Scholar] [CrossRef]
- Fang, P.; Wang, Y.; Wang, M.; Wang, F.; Chi, C.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; et al. Crosstalk between Brassinosteroid and Redox Signaling Contributes to the Activation of CBF Expression during Cold Responses in Tomato. Antioxidants 2021, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. J. Plant Nutr. 2021, 44, 530–545. [Google Scholar] [CrossRef] [Green Version]
- Patterson, B.D.; Macrae, E.A.; Ferguson, I.B. Estimation of Hydrogen Peroxide in Plant Extracts Using Titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenol oxidase in Brta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- He, J.X.; Gendron, J.M.; Sun, Y.; Gampala, S.S.L.; Gendron, N.; Sun, C.Q.; Wang, Z.Y. BZR1 is a transcriptional repressor with dual roles in Brassinosteroid homeostasis and growth responses. Science 2005, 307, 1634–1638. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Fan, X.Y.; Cao, D.M.; Tang, W.Q.; He, K.; Zhu, J.Y.; He, J.X.; Bai, M.Y.; Zhu, S.W.; Oh, E.; et al. Integration of Brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 2010, 19, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.M.; Ji, Y.Y.; Hu, J.; Guo, R.K.; Sun, S.Y.; Wang, X.L. Strigolactones and brassinosteroids antagonistically regulate the stability of D53OsBZR1 complex to determine FC1 expression in rice tillering. Mol. Plant 2020, 13, 586–597. [Google Scholar] [CrossRef]
- Mastoureh, S.; Bernd, M.R.; Salma, B. The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat. Commun. 2016, 7, 12439. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought ’train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef]
- Feng, X.J.; Li, J.R.; Qi, S.L.; Lin, Q.F.; Jin, J.B.; Hua, X.J. Light affects salt stress-induced transcriptional memory of P5CS1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, E8335–E8343. [Google Scholar] [CrossRef] [Green Version]
- Baier, M.; Bittner, A.; Prescher, A.; Van Buer, J. Preparing plants for improved cold tolerance by priming. Plant Cell Environ. 2019, 42, 782–800. [Google Scholar] [CrossRef] [PubMed]
- Crisp, P.A.; Ganguly, D.R.; Smith, A.B.; Murray, K.D.; Estavillo, G.M.; Searle, I.; Ford, E.; Bogdanović, O.; Lister, R.; Borevitz, J.O.; et al. Rapid recovery gene downregulation during excess-light stress and recovery in Arabidopsis. Plant Cell 2017, 29, 1836–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, D.M.; Dennis, E.S.; Finnegan, E.J. The low temperature response pathways for cold acclimation and vernalization are independent. Plant Cell Environ. 2011, 34, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, Q.; Chen, F.D.; Jiang, J.F. Transcriptome analysis reveals Vernalization is independent of cold acclimation in Arabidopsis. BMC Genom. 2021, 22, 462. [Google Scholar] [CrossRef]
- Velitchkova, M.; Popova, A.V.; Faik, A.; Gerganova, M.; Ivanov, A.G. Low temperature and high light dependent dynamic photoprotective strategies in Arabidopsis thaliana. Physiol. Plant 2020, 170, 93–108. [Google Scholar] [CrossRef]
- Amin, B.; Atif, M.J.; Wang, X.; Meng, H.; Ghani, M.I.; Ali, M.; Ding, Y.; Li, X.; Cheng, Z. Effect of low temperature and high humidity stress on physiology of cucumber at different leaf stages. Plant Biol. 2021, 23, 785–796. [Google Scholar] [CrossRef]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.R.; Bäurle, I. Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef] [Green Version]
- He, Y.H.; Li, Z.C. Epigenetic environmental memories in plants: Establishment, maintenance, and reprogramming. Trends Genet. 2018, 34, 856–866. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Brestic, M.; Landi, M.; Skalicky, M. Resistance of Fritillaria imperialis to freezing stress through gene expression, osmotic adjustment and antioxidants. Sci. Rep. 2020, 10, 10427. [Google Scholar] [CrossRef]
- Amin, B.; Atif, M.J.; Meng, H.; Ghani, M.I.; Ali, M.; Wang, X.; Ding, Y.; Li, X.; Cheng, Z. Biochemical and physiological responses of Cucumis sativus Cultivars to different combinations of low temperature and high humidity. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, F.; Song, S.; Yu, X.; Ren, Y.; Zhao, X.; Liu, H.; Liu, G.; Wang, Y.; He, H. CsSWEET2, a hexose transporter from Cucumber (Cucumis sativus L.), affects sugar metabolism and improves cold tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 3886. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shi, J.; Li, M.; Ye, X.; Qi, H. Trehalose triggers hydrogen peroxide and nitric oxide to participate in melon seedlings oxidative stress tolerance under cold stress. Environ. Exp. Bot. 2021, 184, 104379. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Afrin, S.; Khan, M.K.; Hannan, M.A.; Skalicky, M.; Mortuza, M.G.; Brestic, M.; Hossain, M.A.; Murata, Y. Insights into nitric oxide-mediated water balance, antioxidant defence and mineral homeostasis in rice (Oryza sativa L.) under chilling stress. Nitric Oxide 2020, 100, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Sadura, I.; Janeczko, A. Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol. Plant 2018, 62, 601–616. [Google Scholar] [CrossRef] [Green Version]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Kang, J.G.; Gan, Y.T.; Yu, J.H.; Calderón-Urrea, A.; Lyu, J.; Zhang, G.B.; Feng, Z.; Xie, J.M. Transcriptome analysis of pepper (Capsicum annuum) revealed a role of 24-Epibrassinolide in response to chilling. Front. Plant Sci. 2016, 7, 1281. [Google Scholar] [CrossRef] [Green Version]
- Heidari, P.; Entazari, M.; Ebrahimi, A.; Ahmadizadeh, M.; Palumbo, F.; Barcaccia, G. Exogenous EBR ameliorates endogenous hormone contents in tomato species under low-temperature stress. Horticulturae 2021, 7, 84. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.Q.; Miao, L.; Liu, Y.M.; Li, S.Z.; Yu, X.C.; Li, Y.S. 24-Epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef] [Green Version]
- Kawarazaki, T.; Kimura, S.; Iizuka, A.; Hanamata, S.; Nibori, H.; Michikawa, M.; Imai, A.; Abe, M.; Kaya, H.; Kuchitsu, K. A low temperature-inducible protein AtSRC2 enhances the ROS-producing activity of NADPH oxidase AtRbohF. Biochim. Biophys. Acta 2013, 1833, 2775–2780. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.H.; Yang, G.W. Signal function studies of ROS, especially RBOH-sependent ROS, in plant growth, development and environmental stress. J. Plant Growth Regul. 2019, 39, 157–171. [Google Scholar] [CrossRef]
- Fang, P.P.; Yan, M.Y.; Chi, C.; Wang, M.Q.; Zhou, Y.H.; Zhou, J.; Shi, K.; Xia, X.J.; Foyer, C.H.; Yu, J.Q. Brassinosteroids act as a positive regulator of photoprotection in response to chilling stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.; Yu, J.Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef]
- Chen, Z.P.; Xie, Y.J.; Gu, Q.; Zhao, G.; Zhang, Y.H.; Cui, W.T.; Xu, S.; Wang, R.; Shen, W.B. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radical. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Han, J.P.; Köster, P.; Drerup, M.M.; Scholz, M.; Li, S.Z.; Edel, K.H.; Hashimoto, K.; Kuchitsu, K.; Hippler, M.; Kudla, J. Fine-tuning of RBOHF activity is achieved by differential phosphorylation and Ca2+ binding. New Phytol. 2019, 221, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.J.; Fu, X.; Wu, G.X.; Feng, Y.Q.; Li, F.D.; Bi, H.G.; Ai, X.Z. Hydrogen peroxide is involved in hydrogen sulfide-induced carbon assimilation and photoprotection in cucumber seedlings. Environ. Exp. Bot. 2020, 175, 104052. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.C.; Djalovic, I.; Siddique, K.H.M. Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odhiambo, M.O.; Wang, X.C.; Antonio, P.I.J.D.; Shi, Y.Y.; Zhao, B. Effects of Root-Zone temperature on growth, chlorophyll fluorescence characteristics and chlorophyll content of greenhouse pepper plants grown under cold stress in southern China. Russ. Agric. Sci. 2018, 44, 426–433. [Google Scholar] [CrossRef]
- Zhou, R.; Hyldgaard, B.; Yu, X.; Rosenqvist, E.; Ugarte, R.M.A.; Yu, S.; Wu, Z.; Ottosen, C.-O.; Zhao, T. Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 2018, 214, 68. [Google Scholar] [CrossRef]
- Ashrostaghi, T.; Aliniaeifard, S.; Shomali, A.; Azizinia, S.; Koohpalekani, J.A.; Moosavi-Nezhad, M.; Gruda, N.S. Light intensity: The role player in cucumber response to cold stress. Agronomy 2022, 12, 201. [Google Scholar] [CrossRef]
- Tovuu, A.; Zulfugarov, I.S.; Leea, C.H. Correlations between the temperature dependence of chlorophyll fluorescence and the fluidity of thylakoid membranes. Physiol. Plant 2013, 147, 409–416. [Google Scholar] [CrossRef]
- Huang, J.J.; Cheung, P.C.K. Cold stress treatment enhances production of metabolites and biodiesel feedstock in Porphyridium cruentum via adjustment of cell membrane fluidity. Sci. Total Environ. 2021, 780, 146612. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, Q.; Li, Y.; Li, S.; Shi, A.; Zhou, M.; Ren, H.; Yan, Y.; He, C.; Wang, J.; Sun, M.; et al. Photosynthesis Mediated by RBOH-Dependent Signaling Is Essential for Cold Stress Memory. Antioxidants 2022, 11, 969. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11050969
Di Q, Li Y, Li S, Shi A, Zhou M, Ren H, Yan Y, He C, Wang J, Sun M, et al. Photosynthesis Mediated by RBOH-Dependent Signaling Is Essential for Cold Stress Memory. Antioxidants. 2022; 11(5):969. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11050969
Chicago/Turabian StyleDi, Qinghua, Yansu Li, Shuzhen Li, Aokun Shi, Mengdi Zhou, Huazhong Ren, Yan Yan, Chaoxing He, Jun Wang, Mintao Sun, and et al. 2022. "Photosynthesis Mediated by RBOH-Dependent Signaling Is Essential for Cold Stress Memory" Antioxidants 11, no. 5: 969. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox11050969