1. Introduction
Doxorubicin, one of the well-established chemotherapeutic agents, is recommended in the management of breast, liver, kidney ovarian, thyroid cancer, Wilms’ tumor, and acute lymphoblastic and myeloblastic leukemia [
1,
2,
3,
4,
5,
6]. Doxorubicin exhibits its therapeutic activity by inhibiting the enzyme topoisomerase II and cleaving the DNA within tumor cells [
7]. Despite its efficacy in managing the above-mentioned tumors and cancers, doxorubicin pharmacotherapy is limited due to its toxicity, mainly targeting vital organs, viz., the heart, liver, and kidney [
8,
9,
10]. The suspected toxicity could be the outcome of the reactive oxygen species (ROS) generation and other Fenton reactions within the cytoplasm through the doxorubicin metabolite doxorubicinol [
11,
12,
13,
14].
Medicinal plants are being utilized by traditional healers to manage multiple communicable and non-communicable diseases due to the presence of various secondary bioactives, i.e., flavonoids, terpenes, alkaloids, and polyphenols [
15,
16]. Furthermore, due to the H-donating capacities of bioactives, extracts of various medicinal plants neutralize free radicals and terminate Fenton reactions, reducing oxidative stress in the cell [
17]. Since doxorubicin pharmacotherapy is limited due to the free radical generation, it can be assumed that this limitation can be overcome with a secondary agent (medicinal plant with potent antioxidant properties) supplementation to neutralize the doxorubicin-generated free radicals. In addition, it may not only neutralize the doxorubicin-generated free radicals but may also uplift the effectiveness of doxorubicin chemotherapy if it possesses anticancer properties.
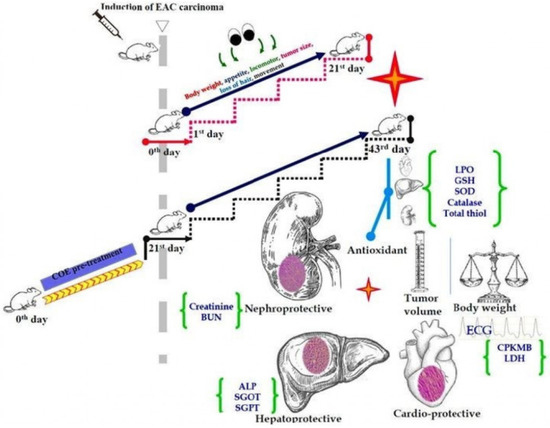
Theobroma cacao L., commonly known as cocoa, belongs to the family Sterculiaceae, which is native to Central America. Its nibs were reported to possess multiple pharmacological spectra due to the presence of various secondary metabolites, i.e., flavonoids, polyphenols, and alkaloids [
18]. Furthermore, the efficacy of cocoa as a free radical scavenger [
19], anti-inflammatory substance [
20], and anticancer agent [
21,
22], and exhibiting cardioprotective [
23], nephroprotective [
24], and hepatoprotective activities [
25], have been reported. Treatment with extracts of cocoa leaves and bark have shown protection against doxorubicin-induced oxidative stress and organ damage (hepatotoxicity, nephrotoxicity, and splenotoxicity) in rats [
26,
27]. In addition, cocoa extract has been reported for its cytotoxic activity against cancer cell lines [
21,
28]. Antioxidants present in cocoa possess free radical scavenging properties and can reduce free radical formation. Although cocoa has been demonstrated to ameliorate doxorubicin-mediated organ toxicity in non-carcinogen animals, its effect on EAC-induced carcinoma has not yet been reported. Hence, we hypothesized that cocoa extract supplementation with doxorubicin could ameliorate organ toxicity and enhance life expectancy. Thus, based on the above hypothesis, the present study was carried out to investigate the effect of hydroalcoholic extract of cocoa bean (COE) supplementation on doxorubicin-induced cardiotoxicity, hepatotoxicity, and nephrotoxicity in EAC mice and to assess its anticancer efficacy, if any, in combination with doxorubicin treatment.
4. Discussion
In our present study, we demonstrated the effects of COE on the heart, liver, and kidney in doxorubicin-treated EAC-bearing mice. Treatment with COE was found to negate the adverse effects of doxorubicin on these organs, as evidenced by significant improvement of the above-mentioned organs’ biomarkers in animals treated with COE in combination with doxorubicin. COE not only reduced the organ toxicity associated with doxorubicin but also acted as an anticancer agent and promoted the anticancer activity of doxorubicin. Treatment with COE alone and in combination with doxorubicin in tumor-bearing mice showed a significant deceleration of cancer progression compared with doxorubicin alone treatment. Additionally, COE alone and in combination with doxorubicin resulted in a significant increase in the MST (33 and 28 days, respectively) compared with doxorubicin alone treatment (25 days) and the EAC group (21 days). These results indicate that COE did not interfere with the anticancer activity of doxorubicin; additionally, it reduced doxorubicin-induced organ toxicity and, therefore, has the potential to serve as a nutraceutical/complementary medicine.
Previously, cardiotoxic, nephrotoxic, and hepatotoxic effects of doxorubicin therapy [
39,
40,
41] and cocoa in preventing multiple organ damage have also been reported [
25,
26,
42]. COE treatment in combination with doxorubicin and pretreatment with the COE showed a significant decrease in the percentage change in body weight and ascites fluid volume in mice compared with the EAC group. Interestingly, we observed a significant decrease in the percentage change in body weight in the COE-pretreated mice compared with doxorubicin, suggesting the prophylactic activity of COE in cancer progression.
Previous, studies have reported that abnormal ECG patterns, such as increased width of the QRS complex, majorly contribute to ventricular hypertrophy, myocardial infarction, altered cardiac function, and other conduction abnormalities [
43,
44]. In the current study, an increase in the width of the QRS complex and a reduction in heart rate was observed in the EAC and doxorubicin groups. However, in all COE-treated groups, abnormal ECG resulting from doxorubicin and EAC was reversed, which suggests a cardioprotective role for COE against doxorubicin and EAC-induced cardiotoxicity.
Alterations in haematological parameters such as myelosuppression and anemia in the EAC mouse model have been well reported [
45]. Similarly, in our present study, analogous findings were observed in EAC-bearing mice. In the treatment groups, both doxorubicin and COE reversed these haematological parameters in EAC-bearing mice; better amelioration was noted in COE group compared with the doxorubicin group, which suggests the beneficial effect of COE over doxorubicin treatment on haematopoiesis. In addition, it has been reported that anemia in EAC mice is caused by iron deficiency (haemolytic and myelopathic conditions), leading to a compromised RBC count [
46]. In contrast, the RBC count in the doxorubicin and COE-treated mice was significantly increased compared with that in the EAC group, suggesting the beneficial role of COE along with doxorubicin treatment.
Elevated CPK-MB and LDH levels are considered important biomarkers of cardiac myocyte damage, especially during the clinical follow-up of doxorubicin therapy. The free radical generation during doxorubicin therapy causes considerable damage to the myocardium, which causes an increase in membrane permeability and thus the release of CPK-MB and LDH [
47]. In the present study, doxorubicin treatment showed 2.54-fold and 5.41-fold increases in CPK-MB and LDH levels, respectively, compared with the normal group. COE treatment alone and in combination with doxorubicin significantly reduced the CPK-MB (2.10-fold and 1.16-fold, respectively) and LDH (3.67-fold and 3.53-fold, respectively) levels, reflecting the cardioprotective activity of COE. Along with these parameters, LPO elevated levels and GSH, SOD, CAT, and total thiol decreased levels in the EAC group were substantially reversed in all COE-treated groups compared with animals treated with doxorubicin alone. This is suggestive of COE scavenging free radicals generated during cancer propagation and doxorubicin therapy, thereby rendering beneficial effects to the host.
Nayagam (2019) [
48] reported that doxorubicin-associated cardiotoxicity due to the accumulation of circulating free fatty acids (FFAs), leading to blockage of the coronary arteries. Cocoa ameliorates the lipid profile in dyslipidaemic conditions in the complex pathogenesis of lipid and glucose metabolism [
49]. These two observations prompted us to assess the effect of COE on lipid-lowering properties in EAC and doxorubicin-induced hyperlipidaemia [
50,
51]. COE alone and in combination with doxorubicin significantly reversed the altered parameters, viz., HDL, cholesterol, and triglycerides, which reflected the shielding role of COE against hyperlipidaemia, which, in turn, prevented cardiac injury and preserved cardiac function.
Hepatocyte damage due to doxorubicin-induced ROS, specifically superoxide anions, compromises mitochondrial function and aggravates liver damage [
52]. Similarly, EAC cells affect the liver through the accumulation of ascetic fluid and by the leakage of liver enzymes such as ALP, SGOT, and SGPT into serum [
53]. Thus, the enzyme content in the liver serves as a biomarker for hepatotoxicity. In addition to the liver, doxorubicin and EAC also contribute to kidney damage. Previously, Mutar (2020) [
54] reported that Ehrlich tumors as a reason for kidney damage by increasing urea and creatinine. These changes in urea and creatinine levels in the kidney contribute to the increased glomerular capillary permeability and tubular atrophy [
55], which are responsible for kidney failure. In our study, the elevation of both hepatic and renal markers in the EAC and doxorubicin groups was observed, which was significantly reversed with COE treatment alone or in combination with doxorubicin. These findings support the beneficial role of COE against EAC- and doxorubicin-associated toxicities and demonstrate its vital role in regulating liver and kidney functions by balancing the serum biomarkers. Furthermore, a COE-mediated antioxidant defensive mechanism was also observed in both liver and kidney tissue. The elevated level of LPO and reduced levels of GSH, CAT, SOD, and thiols observed in both the EAC- and doxorubicin-treated groups were reversed in all COE-treated groups, establishing a beneficial role in cell protection from ROS and reducing organ toxicities concerning the heart, liver, and kidney.
Accumulation of fluid in the intravascular and interstitial spaces in cardiac tissue results in cardiac load, leading to congestion triggered by cardiac edema [
56]. Mishra (2018) [
57] demonstrated that EAC triggers cardiac dysfunction traced by congestion scores. In this study, a higher congestion score in EAC mice was observed compared with the treatment groups. Furthermore, edema was observed in the EAC- and doxorubicin-treated groups, which was reversed in all groups with COE treatment, indicating that COE potentially repaired the cardiac damage caused by doxorubicin and EAC.
Spotty necrosis, venous congestion, sinusoidal congestion, inflammation, and Kupffer cell hyperplasia serve as indicators of hepatic tissue damage [
58,
59]. In the present and previous studies, similar histopathological findings have also been identified in both EAC mice [
60] and doxorubicin-treated animals [
61]. However, in this study, animals treated with DOX alone did not show any improvement in spotty necrosis but showed reduced venous congestion, sinusoidal congestion, and inflammation. Furthermore, the COE in all treated groups showed significant improvement in the reduction of doxorubicin- and EAC-induced hepatic damage. This could be due to the accumulation of lipids and upregulated lipogenesis, as evidenced by upregulated LDL serum levels. The doxorubicin metabolite doxorubicinol is reported to upregulate the ROS system, which may disturb the homeostatic function of hepatocytes and alter multiple biological processes, cellular components, and molecular functions within it, leading to nonalcoholic fatty liver pathogenesis. These reports have been supported by our study. COE treatment was found to reverse EAC- and doxorubicin-induced liver damage. This could be due to potential antioxidant activity owing to the presence of certain biomarkers, such as catchin, (−)-epigallocatechin gallate (EGCG), hirsutrin, hyperoside, and cinaroside (molecules having higher hydrogen donating capacity).
Renal dysfunction induced by cardiac and hepatic injury leads to an increase in renal interstitial pressure on the entire capillary and tubules, which are triggered by an increase in glomerular and tubular congestion [
62]. A similar renal dysfunction with elevated tubular and glomerular congestion has been reported in EAC- and doxorubicin-associatedtoxicities [
63,
64]. These effects could be due to the increase in the ROS system or free radicals and the presence of toxic doxorubicin metabolites in the nephron, which needs to be further investigated. In this study, similar histopathological observations were recorded in the EAC-induced and doxorubicin-treated animals. Interestingly, this damage was reversed in the COE-treated groups, suggesting the beneficial role of COE and nephroprotective activity during doxorubicin treatment in the carcinoma model.
In our study, the Ehrlich ascites model responded better to COE treatment compared with doxorubicin treatment. This may be due to the ascites microenvironment favouring oxidative stress [
65], the proliferation of tumor cells [
66], and comparatively less susceptibility of Ehrlich ascites cells towards oxidative stress [
67]. These factors may also contribute to the reduced chemotherapeutic potential of doxorubicin in ascites tumor models compared with COE, due to its dual advantage of antioxidative and anticancer activities. In addition, COE also attenuate the adverse effects produced by doxorubicin on non-tumor cells without compromising its cancer therapeutic potential. This proves the protective effect of COE and suggesting it be considered as a health supplement during cancer chemotherapy.