Aging-Associated Thyroid Dysfunction Contributes to Oxidative Stress and Worsened Functional Outcomes Following Traumatic Brain Injury
Abstract
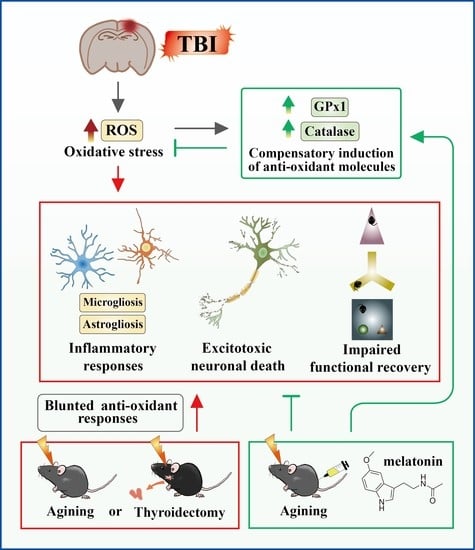
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Designs and Grouping of the Animals
2.3. Animal Model of Traumatic Brain Injury
2.4. Surgical Thyroidectomy
2.5. Neurological Severity Scores
2.6. Object Location Recognition Test
2.7. Spontaneous Alteration in Y-Maze Testing
2.8. Corner Test
2.9. T4 and T3 Measurements
2.10. MDA Measurements
2.11. Measurement of Hydroxyl Free Radicals
2.12. Immunofluorescent Staining
2.13. Real-Time RT-PCR Analysis
2.14. Fluoro-Jade C (FJC) Staining
2.15. Nissl Staining
2.16. Statistical Analysis
3. Results
3.1. Age-Dependent Difference in TBI Susceptibility
3.2. Hypothyroidism Links to TBI-Susceptible Phenotypes
3.3. Excessive Oxidative Stress Links to the TBI-Susceptible Phenotypes
3.4. Feasible Therapeutics for Individuals with Thyroid Dysfunction
4. Discussion
4.1. The Experimental Model with Middle-Aged Animals
4.2. Thyroid Hormone-Dependent Compensatory Protective Events
4.3. Melatonin but Not T3 Provides Therapeutic Benefits in Geriatric TBI
4.4. Thyroid Function as an Adjuvant Biomarker for Outcome Perdition in Geriatric-TBI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, J.; Chan, L.; Flynn, S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021, 102, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.C.; Wang, D.; Ling, G.; Gottesman, R.F.; Selvin, E. Prevalence of Self-Reported Head Injury in the United States. N. Engl. J. Med. 2018, 379, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Whiteneck, G.G.; Cuthbert, J.P.; Corrigan, J.D.; Bogner, J.A. Prevalence of Self-Reported Lifetime History of Traumatic Brain Injury and Associated Disability: A Statewide Population-Based Survey. J. Head Trauma Rehabil. 2016, 31, E55–E62. [Google Scholar] [CrossRef]
- Gardner, R.C.; Dams-O’Connor, K.; Morrissey, M.R.; Manley, G.T. Geriatric Traumatic Brain Injury: Epidemiology, Outcomes, Knowledge Gaps, and Future Directions. J. Neurotrauma 2018, 35, 889–906. [Google Scholar] [CrossRef]
- Thompson, H.J.; McCormick, W.C.; Kagan, S.H. Traumatic brain injury in older adults: Epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 2006, 54, 1590–1595. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Ciceri, L.; Sciutti, F.; Belliato, M.; Iotti, G.A.; Luzzi, S.; Del Maestro, M.; Mezzini, G.; Lafe, E.; et al. Mild head trauma in elderly patients: Experience of an emergency department. Heliyon 2020, 6, e04226. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, J.; Thomas, K.; Waltzman, D.; Sarmiento, K. State-Level Numbers and Rates of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths in 2014. J. Head Trauma Rehabil. 2020, 35, E461–E468. [Google Scholar] [CrossRef]
- Mosenthal, A.C.; Livingston, D.H.; Lavery, R.F.; Knudson, M.M.; Lee, S.; Morabito, D.; Manley, G.T.; Nathens, A.; Jurkovich, G.; Hoyt, D.B.; et al. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J. Trauma 2004, 56, 1042–1048. [Google Scholar] [CrossRef]
- Karr, J.E.; Luoto, T.M.; Gilman, I.G.; Berghem, K.; Kotilainen, A.K.; Iverson, G.L. Age, symptoms, and functional outcome after mild traumatic brain injury. Acta Neurol. Scand. 2020, 141, 183–190. [Google Scholar] [CrossRef]
- Roozenbeek, B.; Maas, A.I.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.B.; Farrer, T.J.; Primosch, M.; Hedges, D.W. Prevalence of traumatic brain injury in the general adult population: A meta-analysis. Neuroepidemiology 2013, 40, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Early, A.N.; Gorman, A.A.; Van Eldik, L.J.; Bachstetter, A.D.; Morganti, J.M. Effects of advanced age upon astrocyte-specific responses to acute traumatic brain injury in mice. J. Neuroinflamm. 2020, 17, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.H.; Stukas, S.; Martens, K.M.; Namjoshi, D.R.; Button, E.B.; Wilkinson, A.; Bashir, A.; Robert, J.; Cripton, P.A.; Wellington, C.L. Age at injury and genotype modify acute inflammatory and neurofilament-light responses to mild CHIMERA traumatic brain injury in wild-type and APP/PS1 mice. Exp. Neurol. 2018, 301, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Pischiutta, F.; Portet, A.; Needham, E.J.; Norton, E.J.; Ferdinand, J.R.; Vegliante, G.; Sammali, E.; Pascente, R.; Caruso, E.; et al. Ageing is associated with maladaptive immune response and worse outcome after traumatic brain injury. Brain Commun. 2022, 4, fcac036. [Google Scholar] [CrossRef]
- Rowe, R.K.; Ziebell, J.M.; Harrison, J.L.; Law, L.M.; Adelson, P.D.; Lifshitz, J. Aging with Traumatic Brain Injury: Effects of Age at Injury on Behavioral Outcome following Diffuse Brain Injury in Rats. Dev. Neurosci. 2016, 38, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Wangler, L.M.; Bray, C.E.; Packer, J.M.; Tapp, Z.M.; Davis, A.C.; O’Neil, S.M.; Baetz, K.; Ouvina, M.; Witzel, M.; Godbout, J.P. Amplified Gliosis and Interferon-Associated Inflammation in the Aging Brain following Diffuse Traumatic Brain Injury. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 9082–9096. [Google Scholar] [CrossRef]
- Baker, D.J.; Petersen, R.C. Cellular senescence in brain aging and neurodegenerative diseases: Evidence and perspectives. J. Clin. Investig. 2018, 128, 1208–1216. [Google Scholar] [CrossRef] [Green Version]
- Tse, K.H.; Herrup, K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech. Ageing Dev. 2017, 161, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Kanu, N.; Penicud, K.; Hristova, M.; Wong, B.; Irvine, E.; Plattner, F.; Raivich, G.; Behrens, A. The ATM cofactor ATMIN protects against oxidative stress and accumulation of DNA damage in the aging brain. J. Biol. Chem. 2010, 285, 38534–38542. [Google Scholar] [CrossRef]
- Matt, S.M.; Johnson, R.W. Neuro-immune dysfunction during brain aging: New insights in microglial cell regulation. Curr. Opin. Pharmacol. 2016, 26, 96–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Li, R.; Yuan, Y.; Zhu, M.; Liu, Y.; Jin, Y.; Yin, Y. PTENalpha is responsible for protection of brain against oxidative stress during aging. FASEB J. 2021, 35, e21943. [Google Scholar] [CrossRef] [PubMed]
- Traina, G.; Cataldi, S.; Siccu, P.; Loreti, E.; Ferri, I.; Sidoni, A.; Codini, M.; Gizzi, C.; Sichetti, M.; Ambesi-Impiombato, F.S.; et al. Mouse Thyroid Gland Changes in Aging: Implication of Galectin-3 and Sphingomyelinase. Mediat. Inflamm. 2017, 2017, 8102170. [Google Scholar] [CrossRef] [Green Version]
- Dedon, J. Thyroid Disease in Aging. Mob. Med. 2022, 119, 351–353. [Google Scholar]
- Rakov, H.; De Angelis, M.; Renko, K.; Hones, G.S.; Zwanziger, D.; Moeller, L.C.; Schramm, K.W.; Fuhrer, D. Aging Is Associated with Low Thyroid State and Organ-Specific Sensitivity to Thyroxine. Thyroid 2019, 29, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Biondi, B.; Cooper, D.S. The clinical significance of subclinical thyroid dysfunction. Endocr. Rev. 2008, 29, 76–131. [Google Scholar] [CrossRef] [Green Version]
- Van den Boogaard, E.; Vissenberg, R.; Land, J.A.; van Wely, M.; Ven der Post, J.A.; Goddijn, M.; Bisschop, P.H. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: A systematic review. Hum. Reprod. Updat. 2016, 22, 532–533. [Google Scholar] [CrossRef] [Green Version]
- Venditti, P.; Napolitano, G.; Di Stefano, L.; Chiellini, G.; Zucchi, R.; Scanlan, T.S.; Di Meo, S. Effects of the thyroid hormone derivatives 3-iodothyronamine and thyronamine on rat liver oxidative capacity. Mol. Cell. Endocrinol. 2011, 341, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.S.; Wang, C.S.; Yeh, C.T.; Lin, K.H. Roles of Thyroid Hormone-Associated microRNAs Affecting Oxidative Stress in Human Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 5220. [Google Scholar] [CrossRef] [Green Version]
- Sagliocchi, S.; Cicatiello, A.G.; Di Cicco, E.; Ambrosio, R.; Miro, C.; Di Girolamo, D.; Nappi, A.; Mancino, G.; De Stefano, M.A.; Luongo, C.; et al. The thyroid hormone activating enzyme, type 2 deiodinase, induces myogenic differentiation by regulating mitochondrial metabolism and reducing oxidative stress. Redox Biol. 2019, 24, 101228. [Google Scholar] [CrossRef]
- Gan, S.; Yang, M.; Fan, L.; Xie, L.; Xu, Y.; Wang, B.; Xu, T.; Yu, L.; Ma, J.; Chen, W. Triiodothyronine Attenuates Silica-Induced Oxidative Stress, Inflammation, and Apoptosis via Thyroid Hormone Receptor alpha in Differentiated THP-1 Macrophages. Chem. Res. Toxicol. 2020, 33, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Cerpa, W. Regulation of Phosphorylated State of NMDA Receptor by STEP(61) Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress. Antioxidants 2021, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shao, C.; Zhang, L.; Siedlak, S.L.; Meabon, J.S.; Peskind, E.R.; Lu, Y.; Wang, W.; Perry, G.; Cook, D.G.; et al. Oxidative Stress Signaling in Blast TBI-Induced Tau Phosphorylation. Antioxidants 2021, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Park, H.Y.; Ali, T.; Kim, M.O. Melatonin as a Potential Regulator of Oxidative Stress, and Neuroinflammation: Mechanisms and Implications for the Management of Brain Injury-Induced Neurodegeneration. J. Inflamm. Res. 2021, 14, 6251–6264. [Google Scholar] [CrossRef]
- Luo, F.; Sandhu, A.F.; Rungratanawanich, W.; Williams, G.E.; Akbar, M.; Zhou, S.; Song, B.J.; Wang, X. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7174. [Google Scholar] [CrossRef]
- Yen, T.L.; Chang, C.C.; Chung, C.L.; Ko, W.C.; Yang, C.H.; Hsieh, C.Y. Neuroprotective Effects of Platonin, a Therapeutic Immunomodulating Medicine, on Traumatic Brain Injury in Mice after Controlled Cortical Impact. Int. J. Mol. Sci. 2018, 19, 1100. [Google Scholar] [CrossRef] [Green Version]
- Shim, H.K.; Kim, S.G.; Kim, T.S.; Kim, S.K.; Lee, S.J. Total Thyroidectomy in the Mouse: The Feasibility Study in the Non-thyroidal Tumor Model Expressing Human Sodium/Iodide Symporter Gene. Nucl. Med. Mol. Imaging 2011, 45, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke A J. Cereb. Circ. 2001, 32, 1005–1011. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.S.; Tsai, P.Y.; Yang, L.Y.; Lecca, D.; Luo, W.; Kim, D.S.; Hoffer, B.J.; Chiang, Y.H.; Greig, N.H.; Wang, J.Y. 3,6’-Dithiopomalidomide Ameliorates Hippocampal Neurodegeneration, Microgliosis and Astrogliosis and Improves Cognitive Behaviors in Rats with a Moderate Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 8276. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Prieur, E.A.K.; Jadavji, N.M. Assessing Spatial Working Memory Using the Spontaneous Alternation Y-maze Test in Aged Male Mice. Bio-Protoc 2019, 9, e3162. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Hsieh, C.Y.; Jayakumar, T.; Tseng, M.F.; Lee, H.N.; Huang, S.W.; Manubolu, M.; Yang, C.H. A Critical Period for the Development of Schizophrenia-Like Pathology by Aberrant Postnatal Neurogenesis. Front. Neurosci. 2019, 13, 635. [Google Scholar] [CrossRef] [PubMed]
- Balkaya, M.; Krober, J.M.; Rex, A.; Endres, M. Assessing post-stroke behavior in mouse models of focal ischemia. J. Cereb. Blood Flow Metab. 2013, 33, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikenari, T.; Kurata, H.; Satoh, T.; Hata, Y.; Mori, T. Evaluation of Fluoro-Jade C Staining: Specificity and Application to Damaged Immature Neuronal Cells in the Normal and Injured Mouse Brain. Neuroscience 2020, 425, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kadar, A.; Wittmann, G.; Liposits, Z.; Fekete, C. Improved method for combination of immunocytochemistry and Nissl staining. J. Neurosci. Methods 2009, 184, 115–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, R.Z.; Lee, K.Y.; Qi, Z.X.; Wang, Z.; Xu, Z.Y.; Wu, X.H.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef]
- Crupi, R.; Cordaro, M.; Cuzzocrea, S.; Impellizzeri, D. Management of Traumatic Brain Injury: From Present to Future. Antioxidants 2020, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Gong, W.; Zhang, D.; Ji, M.; Chen, B.; Chen, B.; Li, X.; Zhou, Y.; Dong, C.; Wen, G.; et al. Ageing related thyroid deficiency increases brain-targeted transport of liver-derived ApoE4-laden exosomes leading to cognitive impairment. Cell Death Dis. 2022, 13, 406. [Google Scholar] [CrossRef]
- Peixoto, M.S.; de Vasconcelos, E.S.A.; Andrade, I.S.; de Carvalho El Giusbi, C.; Coelho Faria, C.; Hecht, F.; Miranda-Alves, L.; Ferreira, A.C.F.; Carvalho, D.P.; Fortunato, R.S. Hypothyroidism induces oxidative stress and DNA damage in breast. Endocr. Relat. Cancer 2021, 28, 505–519. [Google Scholar] [CrossRef]
- Dos Anjos Cordeiro, J.M.; Santos, L.C.; de Oliveira, L.S.; Santos, B.R.; Santos, E.O.; Barbosa, E.M.; de Macedo, I.O.; de Freitas, G.J.C.; Santos, D.A.; de Lavor, M.S.L.; et al. Maternal hypothyroidism causes oxidative stress and endoplasmic reticulum stress in the maternal-fetal interface of rats. Free. Radic. Biol. Med. 2022, 191, 24–39. [Google Scholar] [CrossRef]
- Baldissarelli, J.; Manica, A.; Pillat, M.M.; Bagatini, M.D.; Leal, D.B.R.; Abdalla, F.H.; Morsch, V.M.; Ulrich, H.; Bornemann, C.P.; Chitolina Schetinger, M.R. Increased cytokines production and oxidative stress are related with purinergic signaling and cell survival in post-thyroidectomy hypothyroidism. Mol. Cell. Endocrinol. 2020, 499, 110594. [Google Scholar] [CrossRef]
- Candellone, A.; Saettone, V.; Badino, P.; Girolami, F.; Radice, E.; Bergero, D.; Odore, R.; Meineri, G. Management of Feline Hyperthyroidism and the Need to Prevent Oxidative Stress: What Can We Learn from Human Research? Antioxidants 2021, 10, 1496. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.B.; Riis, K.R.; Winther, K.H.; Larsen, E.L.; Ellervik, C.; Hegedus, L.; Brix, T.H.; Poulsen, H.E.; Bonnema, S.J. Treatment of Hyperthyroidism Reduces Systemic Oxidative Stress, as Measured by Markers of RNA and DNA Damage. J. Clin. Endocrinol. Metab. 2021, 106, e2512–e2520. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Bartalena, L. Role of oxidative stress and selenium in Graves’ hyperthyroidism and orbitopathy. J. Endocrinol. Investig. 2013, 36, 15–20. [Google Scholar]
- Huang, Y.; Fu, T.; Jiao, X.; Liu, S.; Xue, Y.; Liu, J.; Li, Z. Hypothyroidism affects corneal homeostasis and wound healing in mice. Exp. Eye Res. 2022, 220, 109111. [Google Scholar] [CrossRef]
- Villanueva, I.; Alva-Sanchez, C.; Pacheco-Rosado, J. The role of thyroid hormones as inductors of oxidative stress and neurodegeneration. Oxidative Med. Cell. Longev. 2013, 2013, 218145. [Google Scholar] [CrossRef] [Green Version]
- Rao, G.; Verma, R.; Mukherjee, A.; Haldar, C.; Agrawal, N.K. Melatonin alleviates hyperthyroidism induced oxidative stress and neuronal cell death in hippocampus of aged female golden hamster, Mesocricetus auratus. Exp. Gerontol. 2016, 82, 125–130. [Google Scholar] [CrossRef]
- Taussky, P.; Hidalgo, E.T.; Landolt, H.; Fandino, J. Age and salvageability: Analysis of outcome of patients older than 65 years undergoing craniotomy for acute traumatic subdural hematoma. World Neurosurg. 2012, 78, 306–311. [Google Scholar] [CrossRef]
- Lilley, E.J.; Williams, K.J.; Schneider, E.B.; Hammouda, K.; Salim, A.; Haider, A.H.; Cooper, Z. Intensity of treatment, end-of-life care, and mortality for older patients with severe traumatic brain injury. J. Trauma Acute Care Surg. 2016, 80, 998–1004. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-T.; Yen, T.-L.; Chen, Y.-H.; Jan, J.-S.; Teng, R.-D.; Yang, C.-H.; Sun, J.-M. Aging-Associated Thyroid Dysfunction Contributes to Oxidative Stress and Worsened Functional Outcomes Following Traumatic Brain Injury. Antioxidants 2023, 12, 217. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12020217
Hsieh C-T, Yen T-L, Chen Y-H, Jan J-S, Teng R-D, Yang C-H, Sun J-M. Aging-Associated Thyroid Dysfunction Contributes to Oxidative Stress and Worsened Functional Outcomes Following Traumatic Brain Injury. Antioxidants. 2023; 12(2):217. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12020217
Chicago/Turabian StyleHsieh, Cheng-Ta, Ting-Lin Yen, Yu-Hao Chen, Jing-Shiun Jan, Ruei-Dun Teng, Chih-Hao Yang, and Jui-Ming Sun. 2023. "Aging-Associated Thyroid Dysfunction Contributes to Oxidative Stress and Worsened Functional Outcomes Following Traumatic Brain Injury" Antioxidants 12, no. 2: 217. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12020217