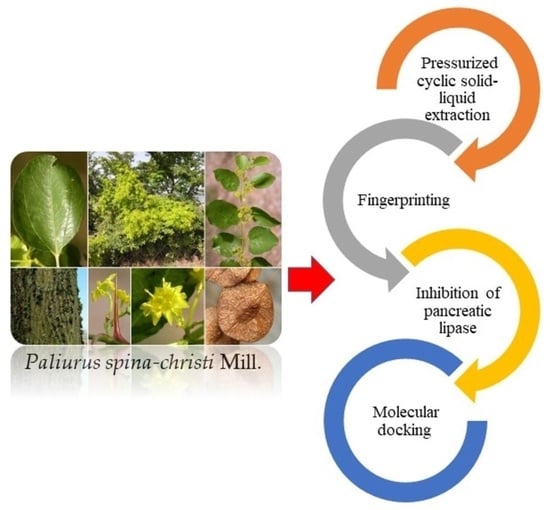
Molecular Docking Studies and In Vitro Activity of Paliurus spina-christi Mill Extracts as Pancreatic Lipase Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of P. spina-christi Extracts
2.3. High Performance Thin Layer Chromatography (HPTLC) Analysis
2.4. 2,2-Diphenyl-1-picrylhidrazyl (DPPH) Photometric Assay
2.5. β-Carotene-Linoleate Bleaching Model System
2.6. Pancreatic Lipase Inhibitory Activity
2.7. Molecular Docking
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Constituents
3.2. Antioxidant Potential
3.3. Pancreatic Lipase Inhibitory Activity
3.4. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, K.; Ito, Y.; Ochiai, J.; Kusuhara, Y.; Hashimoto, S.; Tokudome, S.; Kojima, M.; Wakai, K.; Toyoshima, H.; Tamakoshi, K.; et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac. J. Cancer Prev. 2003, 4, 259–266. [Google Scholar]
- Gey, K.F. Prospects for the Prevention of Free Radical Disease, Regarding Cancer and Cardiovascular Disease. Br. Med. Bull. 1993, 49, 679–699. [Google Scholar] [CrossRef]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K.K. Inhibitors of Pancreatic Lipase: State of the Art and Clinical Perspectives. EXCLI J. 2014, 13, 897. [Google Scholar] [PubMed]
- Li, S.; Pan, J.; Hu, X.; Zhang, Y.; Gong, D.; Zhang, G. Kaempferol Inhibits the Activity of Pancreatic Lipase and Its Synergistic Effect with Orlistat. J. Funct. Foods 2020, 72, 104041. [Google Scholar] [CrossRef]
- Casavecchia, S.; Biscotti, N.; Pesaresi, S.; Biondi, E. The Paliurus Spina-Christi Dominated Vegetation in Europe. Biologia 2015, 70, 879–892. [Google Scholar] [CrossRef]
- Yuca, H.; Özbek, H.; Demirezer, L.Ö.; Güvenalp, Z. Assessment of the α-Glucosidase and α-Amylase Inhibitory Potential of Paliurus Spina-Christi Mill. and Its Terpenic Compounds. Med. Chem. Res. 2022, 31, 1393–1399. [Google Scholar] [CrossRef]
- Sargin, S.A.; Akçicek, E.; Selvi, S. An Ethnobotanical Study of Medicinal Plants Used by the Local People of Alaşehir (Manisa) in Turkey. J. Ethnopharmacol. 2013, 150, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Portale Della Flora d’Italia–Portal to the Flora of Italy. Available online: https://dryades.units.it/floritaly/ (accessed on 4 December 2023).
- Arslan, L.; Kaya, E. Investigation of Antimicrobial and Antioxidant Activities of Paliurus Spina-Christi Mill. in Kahramanmaras, Turkey. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2021, 24, 1161–1169. [Google Scholar] [CrossRef]
- Şen, A. Antioxidant and Anti-Inflammatory Activity of Fruit, Leaf and Branch Extracts of Paliurus Spina-Christi P. Mill. Marmara Pharm. J. 2018, 22, 328–333. [Google Scholar] [CrossRef]
- Takım, K.; Işık, M. Phytochemical Analysis of Paliurus Spina-Christi Fruit and Its Effects on Oxidative Stress and Antioxidant Enzymes in Streptozotocin-Induced Diabetic Rats. Appl. Biochem. Biotechnol. 2020, 191, 1353–1368. [Google Scholar] [CrossRef]
- Zengin, G.; Fernández-Ochoa, Á.; De La Luz Cádiz-Gurrea, M.; Leyva-Jiménez, F.J.; Segura-Carretero, A.; Elbasan, F.; Yildiztugay, E.; Malik, S.; Khalid, A.; Abdalla, A.N.; et al. Phytochemical Profile and Biological Activities of Different Extracts of Three Parts of Paliurus Spina-Christi: A Linkage between Structure and Ability. Antioxidants 2023, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Takım, K. Bioactive Component Analysis and Investigation of Antidiabetic Effect of Jerusalem Thorn (Paliurus Spina-Christi) Fruits in Diabetic Rats Induced by Streptozotocin. J. Ethnopharmacol. 2021, 264, 113263. [Google Scholar] [CrossRef] [PubMed]
- Oguz, F.; Pulat, Ç.Ç.; İlhan, S.; Atmaca, H. GC-MS Analysis and Potential Apoptotic Effect of Paliurus Spina-Christi Mill. Leaf and Flower Extracts against Breast Cancer Cells. Sak. Univ. J. Sci. 2022, 26, 357–364. [Google Scholar] [CrossRef]
- Esfahani, S.M.M.; Tarighi, P.; Dianat, K.; Ashour, T.M.; Mottaghi-Dastjerdi, N.; Aghsami, M.; Sabernavaei, M.; Montazeri, H. Paliurus Spina-Christi Mill Fruit Extracts Improve Glucose Uptake and Activate the Insulin Signaling Pathways in HepG2 Insulin-Resistant Cells. BMC Complement. Med. Ther. 2023, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Brantner, A.H.; Males, Z. Quality Assessment of Paliurus Spina-Christi Extracts. J. Ethnopharmacol. 1999, 66, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Hussain, M.E. Obesity and Diabetes: An Update. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Russo, N.; Chiocchio, I.; Statti, G.; Poli, F.; Conforti, F. Potential Use in the Treatment of Inflammatory Disorders and Obesity of Selected Wild Edible Plants from Calabria Region (Southern Italy). S. Afr. J. Bot. 2020, 128, 304–311. [Google Scholar] [CrossRef]
- Marrelli, M.; Araniti, F.; Statti, G.; Conforti, F. Metabolite Profiling and Biological Properties of Aerial Parts from Leopoldia comosa (L.) Parl.: Antioxidant and Anti-Obesity Potential. S. Afr. J. Bot. 2021, 120, 104–111. [Google Scholar] [CrossRef]
- Egloff, M.P.; Marguet, F.; Buono, G.; Verger, R.; Cambillau, C.; van Tilbeurgh, H. The 2.46 A Resolution Structure of the Pancreatic Lipase-Colipase Complex Inhibited by a C11 Alkyl Phosphonate. Biochemistry 1995, 34, 2751–2762. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1639, 19, 1639–1662. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully Automated Protein-Ligand Interaction Profiler. Nucleic Acids Res. 2015, 43, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zor, M.; Aydin, S.; Güner, N.D.; Başaran, N.; Başaran, A.A. Antigenotoxic Properties of Paliurus Spina-Christi Mill Fruits and Their Active Compounds. BMC Complement. Altern. Med. 2017, 17, 229. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.T.; Abood, K.W.; Ghanim, Q.U.; Ibrahim, N.K.; Al-Naim, A.S. Anti oxidant activity of Paliurus Spina-Christi methanolic extract. Int. J. Bio-Technol. Res. 2013, 3, 1–4. [Google Scholar]
- Aydemir, M.E.; Arslan, A.; Takım, K.; Kılıç Altun, S.; Yılmaz, M.A.; Çakır, O. Inhibitory Effect of Paliurus Spina-Christi Mill., Celtis Tournefortii L. and Nigella Sativa L. on Nε-(Carboxymethyl) Lysine in Meatballs. Meat Sci. 2024, 207, 109362. [Google Scholar] [CrossRef] [PubMed]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Total Phenolic, Flavonoid Contents, and Antioxidant Activities of Fruit, Seed, and Bark Extracts of Zanthoxylum Armatum DC. Sci. World J. 2020, 2020, 8780704. [Google Scholar] [CrossRef]
- Marrelli, M.; Argentieri, M.P.; Avato, P.; Menichini, F.; Conforti, F. Inhibitory Effect on Lipid Absorption and Variability of Chemical Constituents from Capparis sicula subsp. Sicula and Capparis Orientalis. Chem. Biodivers. 2016, 13, 755–761. [Google Scholar] [CrossRef]
- Casacchia, T.; Occhiuzzi, M.A.; Grande, F.; Rizzuti, B.; Granieri, M.C.; Rocca, C.; Gattuso, A.; Garofalo, A.; Angelone, T.; Statti, G. A Pilot Study on the Nutraceutical Properties of the Citrus Hybrid Tacle® as a Dietary Source of Polyphenols for Supplementation in Metabolic Disorders. J. Funct. Foods 2019, 52, 370–381. [Google Scholar] [CrossRef]
- Grande, F.; Occhiuzzi, M.A.; Perri, M.R.; Ioele, G.; Rizzuti, B.; Statti, G.; Garofalo, A. Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy. Molecules 2021, 26, 5718. [Google Scholar] [CrossRef]
- Marrelli, M.; Grande, F.; Occhiuzzi, M.A.; Maione, F.; Mascolo, N.; Conforti, F. Cryptotanshinone and Tanshinone IIA from Salvia Milthorrhiza Bunge (Danshen) as a New Class of Potential Pancreatic Lipase Inhibitors. Nat. Prod. Res. 2021, 35, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Dupont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of Flavonoid and Isoflavonoid Glycosides by Human Small Intestine and Liver β-Glucosidase Activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary Flavonoid and Isoflavone Glycosides Are Hydrolysed by the Lactase Site of Lactase Phlorizin Hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Ioku, K.; Pongpiriyadacha, Y.; Konishi, Y.; Takei, Y.; Nakatani, N.; Terao, J. Beta-Glucosidase Activity in the Rat Small Intestine toward Quercetin Monoglucosides. Biosci. Biotechnol. Biochem. 1998, 62, 1428–1431. [Google Scholar] [CrossRef] [PubMed]






| Sample | IC50 (μg/mL) | ||
|---|---|---|---|
| DPPH | β-Carotene | ||
| 30 min | 60 min | ||
| Leaves | 86.06 ± 1.92 b | 28.21 ± 1.66 b | 43.61 ± 2.51 c |
| Fruits | 53.41 ± 1.24 c | 11.45 ± 0.60 c | 17.38 ± 1.21 d |
| Ascorbic acid * | 2.00 ± 0.01 a | - | - |
| Propyl gallate * | - | 1.00 ± 0.02 a | 1.00 ± 0.02 a |
| Sample | IC50 (mg/mL) |
|---|---|
| Leaf | n.a. |
| Fruit | 2.19 ± 0.02 |
| Orlistat * | 0.018 ± 0.001 |
| Ligand | Structure | BE * | Interactions | |||||
|---|---|---|---|---|---|---|---|---|
| HB *** | *vHPI | π Stacking | ||||||
| Res ** | Distance Å | Donar Angle ° | ||||||
| H-A | D-A | |||||||
| Rutin |  | −8.3 | Asp79 Tyr114 His151 | 2.20 2.57 2.65 | 3.04 3.32 3.43 | 144.05 134.04 136.86 | Phe77 Ile209 Leu213 Phe215 | Phe215 |
| Quercitrin |  | −8.2 | Asp79 Tyr114 His151 | 2.63 3.71 2.54 | 3.04 4.06 3.37 | 107.27 105.56 142.30 | Phe77 Phe215 Ala259 | Phe215 |
| Kaempferol 3-O-glucoside- 7-O-rhamnoside |  | −8.2 | Tyr114 Thr115 Ser152 His263 | 3.02 2.14 2.72 2.44 | 3.98 2.92 3.66 3.25 | 169.28 136.13 164.32 139.61 | Phe77 Tyr114 Phe215 Ala260 | Tyr114 |
| Quercetin |  | −9.8 | His151 Ser152 Arg256 His263 | 3.21 2.27 2.49 2.88 | 3.74 3.06 3.05 3.77 | 114.82 138.20 115.70 153.16 | Phe77 Phe215 Ala 260 Leu264 | Phe215 His263 |
| Keampferol |  | −9.6 | Asp79 His151 Ser152 Arg256 | 2.48 3.10 2.37 2.40 | 3.29 3.65 3.15 2.99 | 140.19 117.11 137.17 117.97 | Phe77 Phe215 Ala 260 Leu264 | His263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, F.; Marrelli, M.; Amodeo, V.; Occhiuzzi, M.A.; Pinzaru, I.; Fucile, M.; Dehelean, C.A.; Alexa, E.; Conforti, F.; Statti, G. Molecular Docking Studies and In Vitro Activity of Paliurus spina-christi Mill Extracts as Pancreatic Lipase Inhibitors. Antioxidants 2024, 13, 160. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13020160
Grande F, Marrelli M, Amodeo V, Occhiuzzi MA, Pinzaru I, Fucile M, Dehelean CA, Alexa E, Conforti F, Statti G. Molecular Docking Studies and In Vitro Activity of Paliurus spina-christi Mill Extracts as Pancreatic Lipase Inhibitors. Antioxidants. 2024; 13(2):160. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13020160
Chicago/Turabian StyleGrande, Fedora, Mariangela Marrelli, Valentina Amodeo, Maria Antonietta Occhiuzzi, Iulia Pinzaru, Mary Fucile, Cristina Adriana Dehelean, Ersilia Alexa, Filomena Conforti, and Giancarlo Statti. 2024. "Molecular Docking Studies and In Vitro Activity of Paliurus spina-christi Mill Extracts as Pancreatic Lipase Inhibitors" Antioxidants 13, no. 2: 160. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13020160