Anti-Inflammatory Effects of Anthocyanin-Enriched Black Soybean Seed Coat (BSSC) Crude Extract on LPS-Induced Acute Liver Injury in Mice
Abstract
:1. Introduction
2. Materials and Methods
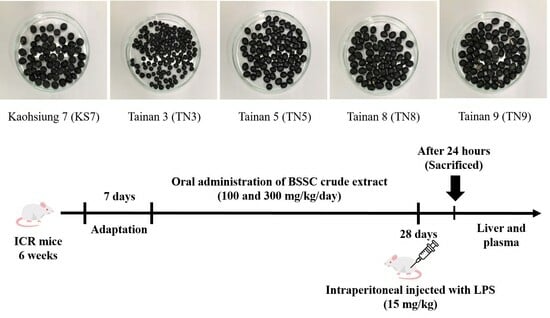
2.1. Extraction of Seed Coats from Different Varieties of Black Soybeans
2.2. Determination of Antioxidant Activity
2.2.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
2.2.2. Superoxide Radical-Scavenging Assay
2.2.3. Reducing Power Assay
2.3. Determination of Total Phenolic Content and Total Anthocyanin Content
2.3.1. Determination of Total Phenolics
2.3.2. Determination of Total Anthocyanins
2.4. Animals and Treatments
2.5. Histopathology Analysis
2.6. Measurement of Antioxidant Enzyme Activity and Lipid Peroxidation in the Liver
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Western Blot Analysis
2.9. Isolation and Purification of Major Compounds
2.10. Anti-Inflammatory Activity Assay
2.11. Statistical Analysis
3. Results
3.1. Total Phenolic and Anthocyanin Contents of the Seed Coat Crude Extracts from Different Black Soybean Varieties
3.2. Antioxidant Activity of the Seed Coat Crude Extracts from Different Black Soybean Varieties
3.3. BSSC Crude Extract Ameliorates Liver Histopathological Changes in LPS-Induced ALI Model Mice
3.4. Effects of BSSC Crude Extract on Antioxidant Enzyme Activity and Lipid Peroxidation in the Liver of LPS-Induced ALI Model Mice
3.5. Effects of BSSC Crude Extract on Proinflammatory and Anti-Inflammatory Cytokine Levels in the Plasma and Liver Tissue of LPS-Induced ALI Model Mice
3.6. Mechanistic Insights into the Protective Effects of BSSC Crude Extracts against ALI in Mice
3.7. Identification and Isolation of the Major Phytochemicals in BSSC Crude Extract and Assessment of Their Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Poll, T. Future of sepsis therapies. Crit. Care 2016, 20, 106. [Google Scholar] [CrossRef]
- Boe, D.M.; Richens, T.R.; Horstmann, S.A.; Burnham, E.L.; Janssen, W.J.; Henson, P.M.; Moss, M.; Vandivier, R.W. Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response. Alcohol. Clin. Exp. Res. 2010, 34, 1723–1732. [Google Scholar] [CrossRef]
- Zhang, Z.; Bagby, G.J.; Stoltz, D.; Oliver, P.; Schwarzenberger, P.O.; Kolls, J.K. Prolonged ethanol treatment enhances lipopolysaccharide/phorbol myristate acetate-induced tumor necrosis factor-α production in human monocytic cells. Alcohol. Clin. Exp. Res. 2006, 25, 444–449. [Google Scholar] [CrossRef]
- Bernal, W. Acute liver failure: Review and update. Int. Anesthesiol. Clin. 2017, 55, 92–106. [Google Scholar] [CrossRef]
- Yun, K.J.; Kim, J.Y.; Kim, J.B.; Lee, K.W.; Jeong, S.Y.; Park, H.J.; Jung, H.J.; Cho, Y.W.; Yun, K.; Lee, K.T. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wu, K.; Wan, J.; Li, L.; Jiang, R.; Jia, M.; Jing, Y.; Zhang, L. Aminotriazole attenuated carbon tetrachloride-induced oxidative liver injury in mice. Food Chem. Toxicol. 2012, 50, 3073–3078. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.L.; Wang, C.J.; Tsai, Y.Y.; Liu, C.L.; Hwang, J.M.; Tseng, T.H. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch. Toxicol. 2000, 74, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A Critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef]
- Choung, M.G.; Baek, I.Y.; Kang, S.T.; Han, W.Y.; Shin, D.C.; Moon, H.P.; Kang, K.H. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.). J. Agric. Food Chem. 2001, 49, 5848–5851. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, N.S.; Shin, S.O.; Shin, S.H.; Lim, S.G.; Suh, D.Y.; Baek, I.Y.; Park, K.Y.; Ha, T.J. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009, 112, 226–231. [Google Scholar] [CrossRef]
- Jin-rui, X.U.; Ming-wei, Z.; Xing-hua, L.I.U.; Zhang-xiong, L.I.U.; Rui-fen, Z.; Ling, S.U.N.; Li-juan, Q.I.U. Correlation between antioxidation and the content of total phenolics and anthocyanin in black soybean accessions. Agric. Sci. China 2007, 6, 150–158. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, L.C.; Ho, S.T.; Tung, Y.T.; Tseng, Y.H.; Wu, J.H. Antioxidant activities and phytochemicals of leaf extracts from 10 native Rhododendron species in Taiwan. Evid.-Based Complement. Alternat. Med. 2014, 2014, 283938. [Google Scholar] [CrossRef] [PubMed]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. J. Food Sci. 1968, 33, 266–274. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F.; Amarowicz, R. Identification and quantification of low molecular weight phenolic antioxidants in seeds of evening primrose (Oenothera biennis L.). J. Agric. Food Chem. 2002, 50, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Park, J.E.; Lee, K.S.; Seo, W.D.; Song, S.B.; Lee, M.H.; Kim, S.; Kim, J.I.; Oh, E.; Pae, S.B.; et al. Identification of anthocyanin compositions in black seed coated Korean adzuki bean (Vigna angularis) by NMR and UPLC-Q-Orbitrap-MS/MS and screening for their antioxidant properties using different solvent systems. Food Chem. 2021, 346, 128882. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S. The inflammatory macrophage: A story of Jekyll and Hyde. Clin. Sci. 2003, 104, 27–38. [Google Scholar] [CrossRef]
- Kim, H.J.; Xu, L.; Chang, K.C.; Shin, S.C.; Chung, J.I.; Kang, D.; Kim, S.H.; Hur, J.A.; Choi, T.H.; Kim, S. Anti-inflammatory effects of anthocyanins from black soybean seed coat on the keratinocytes and ischemia-reperfusion injury in rat skin flaps. Microsurgery 2012, 32, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bing, G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinsons Dis. 2011, 2011, 327089. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Thomas, P.; Breit, S.; Dugas, M.; Mailhammer, R.; van Eden, W.; van der Zee, R.; Biedermann, T.; Prinz, J.; Mack, M.; et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat. Med. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Yao, H.W.; Yue, L. Effect and mechanisms of FR167653, a dual inhibitor of TNF-alpha and IL-1, on BCG plus LPS induced-liver injury. Inflamm. Res. 2005, 54, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Barsig, J.; Kusters, S.; Vogt, K.; Volk, H.D.; Tiegs, G.; Wendel, A. Lipopolysaccharide-induced interleukin-10 in mice: Role of endogenous tumor necrosis factor-alpha. Eur. J. Immunol. 1995, 25, 2888–2893. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.O.; Labuz, D.; Keye, J.; Glauben, R.; Machelska, H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight 2020, 5, e133093. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage polarization and its role in liver disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherratt, P.J.; Huang, H.C.; Yang, C.S.; Pickett, C.B. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J. Biol. Chem. 2003, 278, 4536–4541. [Google Scholar] [CrossRef]
- Yeh, C.H.; Yang, J.J.; Yang, M.L.; Li, Y.C.; Kuan, Y.H. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-kappaB pathway. Free Radic. Biol. Med. 2014, 69, 249–257. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Q.S.; Luo, Q.; Tan, S.; Su, H.; Tang, S.L.; Zhao, Z.L.; Huang, L.P. Simvastatin inhibits apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax. World J. Emerg. Med. 2014, 5, 291–297. [Google Scholar] [CrossRef]
- Pi, J.; Li, T.; Liu, J.; Su, X.; Wang, R.; Yang, F.; Bai, H.; Jin, H.; Cai, J. Detection of lipopolysaccharide induced inflammatory responses in RAW264.7 macrophages using atomic force microscope. Micron 2014, 65, 1–9. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lee, S.U.; Lee, S.; Song, H.H.; Son, T.H.; Kim, Y.U.; Yuk, H.J.; Ro, H.; Lee, C.K.; Hong, S.T.; et al. 3-Methoxy-catalposide inhibits inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Cytokine 2017, 91, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Smythies, L.E.; Mosteller-Barnum, M.; Sibley, D.A.; Russell, M.W.; Merger, M.; Sellers, M.T.; Orenstein, J.M.; Shimada, T.; Graham, M.F.; et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J. Immunol. 2001, 167, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Molonia, M.S.; Occhiuto, C.; Muscara, C.; Speciale, A.; Bashllari, R.; Villarroya, F.; Saija, A.; Cimino, F.; Cristani, M. Cyanidin-3-O-glucoside restores insulin signaling and reduces inflammation in hypertrophic adipocytes. Arch. Biochem. Biophys. 2020, 691, 108488. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966. [Google Scholar] [CrossRef]
- Hyun, K.H.; Gil, K.C.; Kim, S.G.; Park, S.Y.; Hwang, K.W. Delphinidin chloride and its hydrolytic metabolite gallic acid promote differentiation of regulatory T cells and have an anti-inflammatory effect on the allograft model. J. Food Sci. 2019, 84, 920–930. [Google Scholar] [CrossRef]
- Pal, H.C.; Sharma, S.; Strickland, L.R.; Agarwal, J.; Athar, M.; Elmets, C.A.; Afaq, F. Delphinidin reduces cell proliferation and induces apoptosis of non-small-cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLoS ONE 2013, 8, e77270. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Martin-Santamaria, S.; Recio, I.; Sanchez-Moreno, C.; de Pascual-Teresa, B.; Rimbach, G.; de Pascual-Teresa, S. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012, 7, 295–306. [Google Scholar] [CrossRef] [PubMed]







| Varieties | IC50 (μg/mL) | Reducing Power (mg CE/g) | Total Phenolic Content (mg GAE/g) | Total Anthocyanin Content (mg C3GE/g) | |
|---|---|---|---|---|---|
| DPPH Radical | Superoxide Radical | ||||
| Kaohsiung 7 | 3.2 ± 0.3 ab | 35.6 ± 1.0 b | 496.3 ± 16.2 ab | 339.6 ± 23.4 b | 26.5 ± 3.0 b |
| Tainan 3 | 11.3 ± 0.3 c | 68.4 ± 8.9 c | 232.2 ± 24.0 c | 215.8 ± 23.1 c | 6.6 ± 4.9 c |
| Tainan 5 | 3.5 ± 0.2 b | 36.6 ± 1.6 b | 460.2 ± 23.6 b | 327.2 ± 20.4 b | 25.1 ± 1.1 b |
| Tainan 8 | 12.7 ± 0.5 c | 69.8 ± 7.3 c | 176.9 ± 22.2 c | 185.7 ± 29.7 c | 7.3 ± 0.3 c |
| Tainan 9 | 3.0 ± 0.1 ab | 25.1 ± 1.7 ab | 620.8 ± 44.3 a | 437.8 ± 16.9 a | 42.2 ± 3.1 a |
| (+)-Catechin | 1.9 ± 0.02 a | 15.4 ± 2.8 a | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, Y.-T.; Tung, C.-L.; Hsieh, C.-C.; Huang, Y.-C.; Li, S.; Tung, C.-L.; Wu, J.-H. Anti-Inflammatory Effects of Anthocyanin-Enriched Black Soybean Seed Coat (BSSC) Crude Extract on LPS-Induced Acute Liver Injury in Mice. Antioxidants 2024, 13, 311. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13030311
Tung Y-T, Tung C-L, Hsieh C-C, Huang Y-C, Li S, Tung C-L, Wu J-H. Anti-Inflammatory Effects of Anthocyanin-Enriched Black Soybean Seed Coat (BSSC) Crude Extract on LPS-Induced Acute Liver Injury in Mice. Antioxidants. 2024; 13(3):311. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13030311
Chicago/Turabian StyleTung, Yu-Tang, Chun-Liang Tung, Cheng-Chia Hsieh, Yu-Chen Huang, Shiming Li, Chun-Liang Tung, and Jyh-Horng Wu. 2024. "Anti-Inflammatory Effects of Anthocyanin-Enriched Black Soybean Seed Coat (BSSC) Crude Extract on LPS-Induced Acute Liver Injury in Mice" Antioxidants 13, no. 3: 311. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox13030311