Peroxiredoxin 6: The Protector of Male Fertility
Abstract
:1. Introduction
2. PRDX6 and Male Infertility
3. PRDX6 Peroxidase and Phospholipase A2 Activities are Important for Sperm Quality
4. PRDX6 and Sperm Capacitation
5. Perspective and Future Research
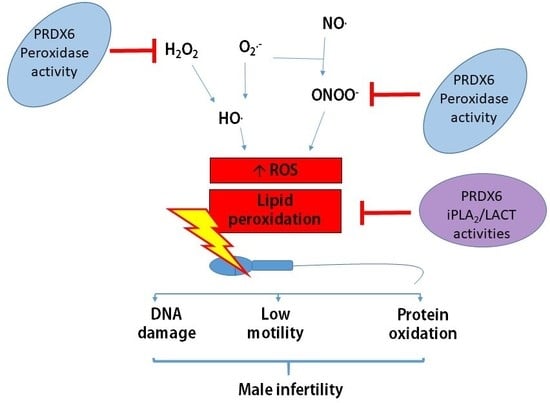
6. Conclusions
Funding
Conflicts of Interest
References
- Bushnik, T.; Cook, J.L.; Yuzpe, A.; Tough, S.; Collins, J. Estimating the prevalence of infertility in Canada. Hum. Reprod. 2012, 7, 738–746. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Towards more objectivity in diagnosis and management of male fertility. Int. J. Androl. 1987, 7, 1–53. [Google Scholar]
- Alvarez, J.G.; Aitken, R.J. Lipid Peroxidation in Human Spermatozoa. In Studies on Men’s Health and Fertility; Agarwal, A., Aitken, R.J., Alvarez, J.G., Eds.; Humana Press: New York, NY, USA, 2012; pp. 119–130. [Google Scholar]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Zini, A.; De Lamirande, E.; Gagnon, C. Reactive oxygen species in semen of infertile patients: Levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int. J. Androl. 1993, 16, 183–188. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Peroxiredoxins: Hidden players in the antioxidant defence of human spermatozoa. Basic Clin. Androl. 2014, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Chabory, E.; Damon, C.; Lenoir, A.; Henry-Berger, J.; Vernet, P.; Cadet, R.; Saez, F.; Drevet, J.R. Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. J. Anim. Sci. 2009, 88, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Frayne, J.; Hall, L. Expression of extracellular glutathione peroxidase type 5 (GPX5) in the rat male reproductive tract. Mol. Hum. Reprod. 1998, 4, 841–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursini, F.; Heim, S.; Kiess, M.; Maiorino, M.; Roveri, A.; Wissing, J.; Flohe, L. Dual function of the selenoprotein phgpx during sperm maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Flohe, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Forster, H.; Boersma, A.; Seiler, A.; Wehnes, H.; Sinowatz, F.; Neumuller, C.; Deutsch, M.J.; Walch, A.; Hrabe de Angelis, M.; et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009, 23, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; de Souza, A.R. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol. Reprod. 2011, 84, 238–247. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. The enzymatic antioxidant system of human spermatozoa. Adv. Androl. 2014, 2014, 1–15. [Google Scholar]
- Gong, S.; San Gabriel, M.; Zini, A.; Chan, P.; O’Flaherty, C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J. Androl. 2012, 33, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Absence of peroxiredoxin 6 amplifies the effect of oxidant stress on mobility and SCSA/CMA3 defined chromatin quality and impairs fertilizing ability of mouse spermatozoa. Biol. Reprod. 2016, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017, 617, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Sorokina, E.M.; Li, H.; Zhou, S.; Raabe, T.; Feinstein, S.I. A novel lysophosphatidylcholine acyl transferase activity is expressed by peroxiredoxin 6. J. Lipid Res. 2016, 31, 292–303. [Google Scholar] [CrossRef] [PubMed]
- McDougal, W.; Wein, A.; Kavoussi, L.; Novick, A.; Partin, A.; Peters, C.; Ramchandani, P. Campbell-Walsh Urology, 10th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 activates maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Limphong, P.; Pieper, J.; Liu, Q.; Rodesch, C.K.; Christians, E.; Benjamin, I.J. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012, 26, 1442–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkosem, B.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 2015, 5, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Robaire, B. Ageing of the male germ line. Nat. Rev. Urol. 2013, 10, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Addai, J.; Smith, R.P.; Coward, R.M.; Lamb, D.J.; Lipshultz, L.I. The effects of advanced paternal age on fertility. Asian J. Androl. 2013, 15, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, T.G.; Aston, K.I.; Meyer, T.; Carrell, D.T. The Sperm Epigenome, Male Aging, and Potential Effects on the Embryo. In The Male Role in Pregnancy Loss and Embryo Implantation Failure; Bronson, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 81–93. [Google Scholar]
- Conti, S.; Eisenberg, M. Paternal aging and increased risk of congenital disease, psychiatric disorders, and cancer. Asian J. Androl. 2016, 18, 420–424. [Google Scholar] [PubMed]
- Ryu, D.-Y.; Kim, K.-U.; Kwon, W.-S.; Rahman, M.S.; Khatun, A.; Pang, M.-G. Peroxiredoxin activity is a major landmark of male fertility. Sci. Rep. 2017, 7, 17174. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.R.; Fernandez, M.C.; Scarlata, E.; Dodia, C.; Feinstein, S.I.; Fisher, A.B.; O’Flaherty, C. Deficiency of peroxiredoxin 6 or inhibition of its phospholipase A2 activity impair the in vitro sperm fertilizing competence in mice. Sci. Rep. 2017, 7, 12994. [Google Scholar] [CrossRef] [PubMed]
- Robaire, B.; Hinton, B.T.; Orgebin-Crist, M.C. The Epididymis. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neill, J.D., Plant, T.M., Pfaff, D.W., Challis, J.R.G., de Kretser, D.M., Richards, J.S., Wassarman, P.M., Eds.; Academic Press: St Louis, MO, USA, 2006; pp. 1071–1148. [Google Scholar]
- Aitken, R.J.; Buckingham, D.W.; Carreras, A.; Stewart Irvine, D. Superoxide dismutase in human sperm suspensions: Relationship with cellular composition, oxidative stress, and sperm function. Free Radic. Biol. Med. 1996, 21, 495–504. [Google Scholar] [CrossRef]
- Liu, Y.; O’Flaherty, C. In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality. Asian J. Androl. 2017, 19, 73–79. [Google Scholar] [PubMed]
- Sullivan, R.; Frenette, G.; Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 2007, 9, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Thimon, V.; Frenette, G.; Saez, F.; Thabet, M.; Sullivan, R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: A proteomic and genomic approach. Hum. Reprod. 2008, 23, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Matsushita-Fournier, D.; O’Flaherty, C. Oxidative Stress Induces Redox-Dependent Modifications of Human Sperm and Seminal Plasma Proteins and Damages the Paternal Genome; McGill University: Montreal, QC, Canada, 2015. [Google Scholar]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992, 13, 368–378. [Google Scholar] [PubMed]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J. Androl. 1992, 13, 379–386. [Google Scholar] [PubMed]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ng, S.C. Fertilizing ability of DNA-damaged spermatozoa. J. Exp. Zool. 1999, 284, 696–704. [Google Scholar] [CrossRef]
- Fatehi, A.N.; Bevers, M.M.; Schoevers, E.; Roelen, B.A.J.; Colenbrander, B.; Gadella, B.M. DNA damage in bovine sperm does not block fertilization and early embryonic development but induces apoptosis after the first cleavages. J. Androl. 2006, 27, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kino, K.; Sugiyama, H. Gc→Cg transversion mutation might be caused by 8-oxoguanine oxidation product. Nucleic Acids Symp. Ser. 2000, 44, 139–140. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Beorlegui, N.; Beconi, M. Role of Superoxide Anion and Hydrogen Peroxide in Bovine Acrosome Reaction. In Andrology in the 21st Century, Proceedings of the VII International Congress of Andrology, Montreal, QC, Canada, 15–19 June 2001; Robaire, B., Chemes, H., Morales, C., Eds.; Medimond Publications: Englewood, NJ, USA, 2001; pp. 103–108. [Google Scholar]
- De Lamirande, E.; O’Flaherty, C. Sperm Capacitation as an Oxidative Event. In Studies on Men’s Health and Fertility, Oxidative Stress in Applied Basic Research and Clinical Practice; Aitken, J., Alvarez, J., Agawarl, A., Eds.; Springer Science: Berlin, Germany, 2012; pp. 57–94. [Google Scholar]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic. Biol. Med. 2006, 40, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Moawad, A.; Morielli, T.; Fernandez, M.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: Modulation and protein kinase A dependency. Mol. Hum. Reprod. 2004, 10, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [PubMed]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol. Reprod. 1996, 55, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Cormier, N.; Sirard, M.A.; Bailey, J.L. Premature capacitation of bovine spermatozoa is initiated by cryopreservation. J. Androl. 1997, 18, 461–468. [Google Scholar] [PubMed]
- Florman, H.M.; Fissore, R.A. Chapter 4—Fertilization in Mammals. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 149–196. [Google Scholar]
- Ozkosem, B.; Moawad, A.; O’Flaherty, C. 228—Peroxiredoxin 6 is involved in the acquisition of fertilizing competence of mouse spermatozoa. Free Radic. Biol. Med. 2015, 87 (Suppl. 1), S104. [Google Scholar] [CrossRef]
- Fisher, A.B.; Vasquez-Medina, J.P.; Dodia, C.; Sorokina, E.M.; Tao, J.Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2017, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Benipal, B.; Zhou, S.; Dodia, C.; Chatterjee, S.; Tao, J.Q.; Sorokina, E.M.; Raabe, T.; Feinstein, S.I.; Fisher, A.B. Critical role of peroxiredoxin 6 in the repair of peroxidized cell membranes following oxidative stress. Free Radic. Biol. Med. 2015, 87, 356–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarpotto, E.; Domenicotti, C.; Paola, D.; Vitali, A.; Nitti, M.; Pronzato, M.A.; Biasi, F.; Cottalasso, D.; Marinari, U.M.; Dragonetti, A.; et al. Regulation of rat hepatocyte protein kinase c beta isoenzymes by the lipid peroxidation product 4-hydroxy-2,3-nonenal: A signaling pathway to modulate vesicular transport of glycoproteins. Hepatology 1999, 29, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Pronzato, M.A.; Domenicotti, C.; Biasi, F.; Chiarpotto, E.; Cottalasso, D.; Viotti, P.; Melloni, E.; Marinari, U.M.; Poli, G. Inactivation of hepatocyte protein kinase C by carbon tetrachloride: Involvement of drug’s metabolic activation and prooxidant effect. Biochem. Biophys. Res. Commun. 1990, 171, 1353–1360. [Google Scholar] [CrossRef]
- Sampey, B.P.; Carbone, D.L.; Doorn, J.A.; Drechsel, D.A.; Petersen, D.R. 4-hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (ERK) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol. Pharmacol. 2007, 71, 871–883. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Gagnon, C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol. Hum. Reprod. 2002, 8, 124–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J.; et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Invest. 2009, 119, 2074–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.; Moreno, S.G.; Sinowatz, F.; Ursini, F.; Kolle, S.; Roveri, A.; Brielmeier, M.; Wurst, W.; Maiorino, M.; Bornkamm, G.W. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol. Cell Biol. 2005, 25, 7637–7644. [Google Scholar] [CrossRef] [PubMed]
- Noblanc, A.; Peltier, M.; Damon-Soubeyrand, C.; Kerchkove, N.; Chabory, E.; Vernet, P.; Saez, F.; Cadet, R.; Janny, L.; Pons-Rejraji, H.; et al. Epididymis response partly compensates for spermatozoa oxidative defects in snGPx4 and GPx5 double mutant mice. PLoS ONE 2012, 7, e38565. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Dionne, L.; Guo, Q.; Kumar, T.R.; Lebovitz, R.M. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 1998, 139, 4008–4011. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Gargano, M.; Cao, J.; Bronson, R.T.; Heimler, I.; Hutz, R.J. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J. Biol. Chem. 1998, 273, 7765–7769. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox7120173
O’Flaherty C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants. 2018; 7(12):173. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox7120173
Chicago/Turabian StyleO’Flaherty, Cristian. 2018. "Peroxiredoxin 6: The Protector of Male Fertility" Antioxidants 7, no. 12: 173. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox7120173