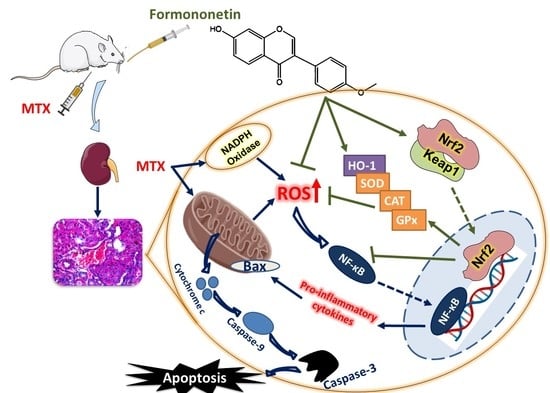
Formononetin Upregulates Nrf2/HO-1 Signaling and Prevents Oxidative Stress, Inflammation, and Kidney Injury in Methotrexate-Induced Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Treatments
2.2. Biochemical Assays
2.2.1. Determination of Creatinine and Urea
2.2.2. Determination of Kim-1, Caspases and Cytokines
2.2.3. Determination of Oxidative Stress Markers and Antioxidants
2.2.4. Determination of HO-1 Activity
2.2.5. Determination of ATP
2.3. Histological Examination of Kidney Sections
2.4. Gene Expression Analysis
2.5. Western Blotting
2.6. Assessment of the Impact of FN on MTX Cytotoxicity in HepG-2 Cells
2.7. Statistical Analysis
3. Results:
3.1. FN Prevents Renal Dysfunction and Injury in MTX-Administered Rats
3.2. FN Prevents Oxidative Stress and DNA Damage in MTX-Induced Rats
3.3. FN Upregulates Nrf2/HO-1 Signaling in Kidney of MTX-Administered Rats
3.4. FN Increases Renal ATP Levels in MTX-Induced Rats
3.5. FN Suppresses Renal Inflammation in MTX-Induced Rats
3.6. FN Prevents MTX-Induced Apoptosis
3.7. FN Does Not Interfere with the Antitumor Activity of MTX
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kremer, J.M. Toward a better understanding of methotrexate. Arthritis Rheum. 2004, 50, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Carson, K.R.; Butler, S.; Liu, W.; Bartlett, N.L.; Wagner-Johnston, N.D. High incidence of methotrexate associated renal toxicity in patients with lymphoma: A retrospective analysis. Leuk. Lymphoma 2014, 55, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Widemann, B.C.; Adamson, P.C. Understanding and managing methotrexate nephrotoxicity. Oncologist 2006, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Abd El-Twab, S.M. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of ppargamma and nrf2: Protective effect of 18beta-glycyrrhetinic acid. Chem.-Biol. Interact. 2017, 270, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Małyszko, J.; Kozłowska, K.; Kozłowski, L.; Małyszko, J. Nephrotoxicity of anticancer treatment. Nephrol. Dial. Transplant. 2016, 32, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Twab, S.M.; Hozayen, W.G.; Hussein, O.E.; Mahmoud, A.M. 18 β-glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the nrf2/are/ho-1 pathway and endogenous antioxidants. Ren. Fail. 2016, 38, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Germoush, M.O.; Al-Anazi, K.M.; Mahmoud, A.H.; Farah, M.A.; Allam, A.A. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating nrf2/are/ho-1 signaling. Biomed. Pharmacother. 2018, 106, 499–509. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing nf-κb/nlrp3 inflammasome activation and up-regulating nrf2/are/ho-1 signaling. Inflamm. Res. 2019, 68, 511–523. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Maghrabi, I.A. Camel milk attenuates methotrexate-induced kidney injury via activation of pi3k/akt/enos signaling and intervention with oxidative aberrations. Food Funct. 2018, 9, 2661–2672. [Google Scholar] [CrossRef]
- Kolli, V.K.; Abraham, P.; Isaac, B.; Selvakumar, D. Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy 2009, 55, 83–90. [Google Scholar] [CrossRef]
- Abdel-Raheem, I.T.; Khedr, N.F. Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexate-induced kidney damage in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Loverre, A.; Ditonno, P.; Crovace, A.; Gesualdo, L.; Ranieri, E.; Pontrelli, P.; Stallone, G.; Infante, B.; Schena, A.; Di Paolo, S.; et al. Ischemia-reperfusion induces glomerular and tubular activation of proinflammatory and antiapoptotic pathways: Differential modulation by rapamycin. J. Am. Soc. Nephrol. JASN 2004, 15, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Ahmadi, A.; Mohammadi, H.; Ommati, M.M.; Azarpira, N.; Niknahad, H. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed. Pharmacother. 2018, 107, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The role of nrf2 in cardiovascular function and disease. Oxid. Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by nrf2 through binding to the amino-terminal neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of nrf2 and pparγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hozayen, W.G.; Ramadan, S.M. Berberine ameliorates methotrexate-induced liver injury by activating nrf2/ho-1 pathway and pparγ, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother. 2017, 94, 280–291. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Mohammed, H.M.; Khadrawy, S.M.; Galaly, S.R. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of nrf2/are/ho-1, ppargamma and tgf-beta1/smad3 signaling, and amelioration of oxidative stress and inflammation. Chem.-Biol. Interact. 2017, 277, 146–158. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Zaki, A.R.; Hassan, M.E.; Mostafa-Hedeab, G. Commiphora molmol resin attenuates diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis by modulating oxidative stress, inflammation, angiogenesis and nrf2/are/ho-1 signaling. Chem.-Biol. Interact. 2017, 270, 41–50. [Google Scholar] [CrossRef]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription factor nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef] [PubMed]
- ALHaithloul, H.A.S.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates nrf2/are/ho-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmacother. 2019, 111, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, J.H.; Ko, J.-H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Formononetin regulates multiple oncogenic signaling cascades and enhances sensitivity to bortezomib in a multiple myeloma mouse model. Biomolecules 2019, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wan, C.; Li, W.; Yao, L.; Zhao, H.; Zou, Y.; Peng, D.; Huang, W. Formononetin protects against acetaminophen-induced hepatotoxicity through enhanced nrf2 activity. PLoS ONE 2017, 12, e0170900. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ji, W.; Fu, Q.; Ma, S. Formononetin inhibited the inflammation of lps-induced acute lung injury in mice associated with induction of ppar gamma expression. Inflammation 2013, 36, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Arya, D.; Singh, S. Formononetin attenuates isoproterenol-induced cardiac toxicity in rats owing to its antioxidant, anti-inflammatory and anti-apoptotic activity. FASEB J. 2019, 33, lb399. [Google Scholar]
- Huang, D.; Wang, C.; Duan, Y.; Meng, Q.; Liu, Z.; Huo, X.; Sun, H.; Ma, X.; Liu, K. Targeting oct2 and p53: Formononetin prevents cisplatin-induced acute kidney injury. Toxicol. Appl. Pharmacol. 2017, 326, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, Y.; Gao, L.; Yin, H.; Xie, Z.; Wang, D.; Zhu, Z.; Han, X. Formononetin attenuates il-1β-induced apoptosis and nf-κb activation in ins-1 cells. Molecules 2012, 17, 10052–10064. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci. 2019, 219, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K. Creatinine assay in the presence of protein with lkb 8600 reaction rate analyser. Clin. Chim. Acta Int. J. Clin. Chem. 1972, 38, 475–476. [Google Scholar]
- Coulombe, J.J.; Favreau, L. A new simple semimicro method for colorimetric determination of urea. Clin. Chem. 1963, 9, 102–108. [Google Scholar] [PubMed]
- Hozayen, W.G.; Mahmoud, A.M.; Desouky, E.M.; El-Nahass, E.-S.; Soliman, H.A.; Farghali, A.A. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ros production and redox imbalance in wistar rats. Biomed. Pharmacother. 2019, 109, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15n]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Matkovics, B.; Szabo, L.; Varga, I.S. Determination of enzyme activities in lipid peroxidation and glutathione pathways (in hungarian). Laboratoriumi Diagnosztika 1998, 15, 248–249. [Google Scholar]
- Abraham, N.G.; Lutton, J.D.; Levere, R.D. Heme metabolism and erythropoiesis in abnormal iron states: Role of delta-aminolevulinic acid synthase and heme oxygenase. Exp. Hematol. 1985, 13, 838–843. [Google Scholar]
- Mahmoud, A.M. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of pparγ and abrogation of oxidative stress and inflammation. Can. J. Physiol. Pharm. 2014, 92, 717–724. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.M.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Mahmoud, A.M. Gamma-glutamylcysteine ethyl ester protects against cyclophosphamide-induced liver injury and hematologic alterations via upregulation of ppargamma and attenuation of oxidative stress, inflammation, and apoptosis. Oxid. Med. Cell. Longev. 2016, 2016, 4016209. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar] [PubMed]
- Salazar, J.H. Overview of urea and creatinine. Lab. Med. 2014, 45, e19–e20. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. Annu. Rev. Pharm. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Moresco, R.N.; Bochi, G.V.; Stein, C.S.; de Carvalho, J.A.M.; Cembranel, B.M.; Bollick, Y.S. Urinary kidney injury molecule-1 in renal disease. Clin. Chim. Acta 2018, 487, 15–21. [Google Scholar] [CrossRef]
- Huang, D.; Wang, C.; Meng, Q.; Liu, Z.; Huo, X.; Sun, H.; Yang, S.; Ma, X.; Peng, J.; Liu, K. Protective effects of formononetin against rhabdomyolysis-induced acute kidney injury by upregulating nrf2 in vivo and in vitro. RSC Adv. 2016, 6, 110874–110883. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Abd El-Twab, S.M.; Hozayen, W.G. Ferulic acid protects against methotrexate nephrotoxicity via activation of nrf2/are/ho-1 signaling and pparγ, and suppression of nf-κb/nlrp3 inflammasome axis. Food Funct. 2019, 10, 4593–4607. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. Ros function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Moreno, J.A.; Santamaria, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Nf-κb in renal inflammation. J. Am. Soc. Nephrol. 2010, 21, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- López-Franco, O.; Suzuki, Y.; Sanjuán, G.; Blanco, J.; Hernández-Vargas, P.; Yo, Y.; Kopp, J.; Egido, J.; Gómez-Guerrero, C. Nuclear factor-κb inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am. J. Pathol. 2002, 161, 1497–1505. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, G.; Zheng, X.; Wang, W.; Ling, Y.; Tang, H.; Zhang, J. Neuroprotective effect of formononetin against tbi in rats via suppressing inflammatory reaction in cortical neurons. Biomed. Pharmacother. 2018, 106, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, D.; Kang, K.; Song, J.; Choi, Y.-K. Inhibition of intracellular ros accumulation by formononetin attenuates cisplatin-mediated apoptosis in llc-pk1 cells. Int. J. Mol. Sci. 2018, 19, 813. [Google Scholar] [CrossRef]
- Lee, C. Collaborative power of nrf2 and pparγ activators against metabolic and drug-induced oxidative injury. Oxid. Med. Cell. Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; Jones, A.M.; Wilkinson, J.A.; Romero, M.; Duarte, J.; Alexander, M.Y. A novel role for small molecule glycomimetics in the protection against lipid-induced endothelial dysfunction: Involvement of akt/enos and nrf2/are signaling. Biochim. Biophys. Acta 2017, 1861, 3311–3322. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; McCarthy, E.M.; Moreno-Martinez, D.; Langford-Smith, A.; Romero, M.; Duarte, J.; Alexander, M.Y. Endothelial microparticles prevent lipid-induced endothelial damage via akt/enos signaling and reduced oxidative stress. FASEB J. 2017, 31, 4636–4648. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between nrf2 and nf-κb response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Pan, H.; Wang, H.; Wang, X.; Zhu, L.; Mao, L. The absence of nrf2 enhances nf-b-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat. Inflamm. 2012, 2012, 217580. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xiao, Q.; Zhou, J.; Feng, H.; Liu, G.; Ci, X. Licochalcone a upregulates nrf2 antioxidant pathway and thereby alleviates acetaminophen-induced hepatotoxicity. Front. Pharmacol. 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, X.; Zhang, J.; Zeng, G.; Zhao, H.; Liu, Y.; Qiu, R.; Mo, L.; Ye, Y. Formononetin protects tbi rats against neurological lesions and the underlying mechanism. J. Neurol. Sci. 2014, 338, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.W. Dexrazoxane for the treatment of chemotherapy-related side effects. Cancer Manag. Res. 2014, 6, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-M.; Xu, T.-M.; Zhao, Y.-H.; Wang, Y.-C.; Cui, M.-h. In vitro and in vivo anti-cancer activity of formononetin on human cervical cancer cell line hela. Tumor Biol. 2014, 35, 2279–2284. [Google Scholar] [CrossRef] [PubMed]









| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| NRF2 | TTGTAGATGACCATGAGTCGC | TGTCCTGCTGTATGCTGCTT |
| HO-1 | GTAAATGCAGTGTTGGCCCC | ATGTGCCAGGCATCTCCTTC |
| SOD | ACACCTATGCACTCCACAGAC | ACATTCGACCTCTGGGGGTA |
| CAT | GCGGGAACCCAATAGGAGAT | CAGGTTAGGTGTGAGGGACA |
| TNFa | AAATGGGCTCCCTCTCATCAGTTC | TCTGCTTGGTGGTTTGCTACGAC |
| IL-1b | GACTTCACCATGGAACCCGT | GGAGACTGCCCATTCTCGAC |
| COX2 | TGATCTACCCTCCCCACGTC | ACACACTCTGTTGTGCTCCC |
| NOS2 | ATTCCCAGCCCAACAACACA | GCAGCTTGTCCAGGGATTCT |
| BAX | AGGACGCATCCACCAAGAAG | CAGTTGAAGTTGCCGTCTGC |
| BCL2 | ACTCTTCAGGGATGGGGTGA | TGACATCTCCCTGTTGACGC |
| Casp3 | GAGCTTGGAACGCGAAGAAA | TAACCGGGTGCGGTAGAGTA |
| Actb | AGGAGTACGATGAGTCCGGC | CGCAGCTCAGTAACAGTCCG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aladaileh, S.H.; Hussein, O.E.; Abukhalil, M.H.; Saghir, S.A.M.; Bin-Jumah, M.; Alfwuaires, M.A.; Germoush, M.O.; Almaiman, A.A.; Mahmoud, A.M. Formononetin Upregulates Nrf2/HO-1 Signaling and Prevents Oxidative Stress, Inflammation, and Kidney Injury in Methotrexate-Induced Rats. Antioxidants 2019, 8, 430. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8100430
Aladaileh SH, Hussein OE, Abukhalil MH, Saghir SAM, Bin-Jumah M, Alfwuaires MA, Germoush MO, Almaiman AA, Mahmoud AM. Formononetin Upregulates Nrf2/HO-1 Signaling and Prevents Oxidative Stress, Inflammation, and Kidney Injury in Methotrexate-Induced Rats. Antioxidants. 2019; 8(10):430. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8100430
Chicago/Turabian StyleAladaileh, Saleem H., Omnia E. Hussein, Mohammad H. Abukhalil, Sultan A. M. Saghir, May Bin-Jumah, Manal A. Alfwuaires, Mousa O. Germoush, Amer A. Almaiman, and Ayman M. Mahmoud. 2019. "Formononetin Upregulates Nrf2/HO-1 Signaling and Prevents Oxidative Stress, Inflammation, and Kidney Injury in Methotrexate-Induced Rats" Antioxidants 8, no. 10: 430. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8100430