Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical Reagents and Instrumentation
2.3. Extraction Procedures
2.4. Chemical Characterization and Isolation of the Flavanones
2.5. Determination of Total Phenolic Content
2.6. 2,2′-diphenyl-1-picrylhydrazyl (DPPH) Assay
2.7. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Diammonium Salt (ABTS) Assay
2.8. Cell Cultures
2.9. Inhibition of Nitric Oxide (NO) Production in Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Cells
2.10. Cell Viability Assay
2.11. Immuno-Fluorescence Monitoring Nuclear Factor Kappa B (NF-kB) Translocation
2.12. Real-Time RT-PCR Assays
2.13. Immunoblotting Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Extracts and Characterization of the Isolated Compounds
3.2. Antioxidant Profile of Glycyrrhiza glabra L. Leaf Extracts and Their Flavanone-Components
3.3. Flavanones from Glycyrrhiza glabra L. Leaf Extracts Affect NO Production in LPS-Stimulated RAW 264.7 Cell Line
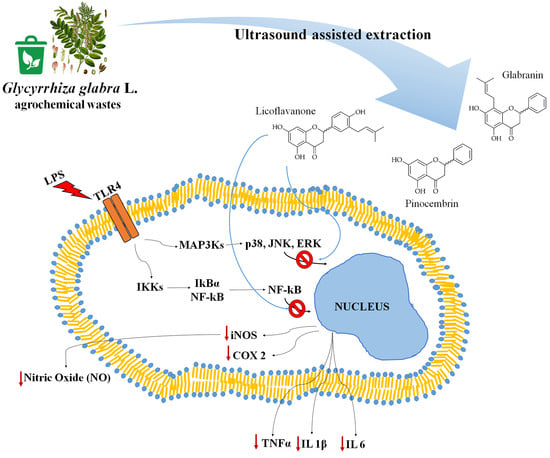
3.4. Licoflavanone Exerts Anti-Inflammatory Effects by Reducing NF-kB Nuclear Translocation
3.5. Licoflavanone Disrupts MAPK/NF-kB Pathway and Modulates Pro-Inflammatory Cytokines
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.; Badolato, M.; Pessina, F.; Sticozzi, C.; Maestrini, V.; Aldinucci, C.; Luongo, L.; Guida, F.; Ligresti, A.; Artese, A.; et al. Design and synthesis of new transient receptor potential vanilloid type-1 (TRPV1) channel modulators: Identification, molecular modeling analysis, and pharmacological characterization of the N-(4-hydroxy-3-methoxybenzyl)-4-(thiophen-2-yl)butanamide, a small molecule endowed with agonist TRPV1 activity and protective effects against oxidative stress. ACS Chem. Neurosci. 2016, 7, 737–748. [Google Scholar] [PubMed]
- Sales, T.A.; Teodorico, S.M.; Ramalho, C. Current anti-inflammatory therapies and the potential of secretory Phospholipase A2 inhibitors in the design of new anti-inflammatory drugs: A review of 2012–2018. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Galligano, F.; Aiello, F. Structure–activity relationships for the synthesis of selective cyclooxygenase 2 inhibitors: An overview (2009–2016). MedChemComm 2017, 8, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Coricello, A.; El-Magboub, A.; Luna, M.; Ferrario, A.; Haworth, I.S.; Gomer, C.J.; Aiello, F.; Adams, J.D. Rational drug design and synthesis of new α-Santonin derivatives as potential COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Frattaruolo, L.; Haworth, I.; Brindisi, M.; El-magboub, A.; Ferrario, A.; Gomer, C.; Aiello, F.; Adams, J.D. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon 2019, 5, e01366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and Anti-Inflammatory Properties of Nigella sativa Oil in Human Pre-Adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhasvar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatoy Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Cappello, A.R.; Dolce, V.; Iacopetta, D.; Martello, M.; Fiorillo, M.; Curcio, R.; Muto, L.; Dhanyalayam, D. Bergamot (Citrus bergamia Risso) Flavonoids and Their Potential Benefits in Human Hyperlipidemia and Atherosclerosis: An Overview. Mini Rev. Med. Chem. 2016, 16, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza spp. And Its Bioactive Constituents: Update and Review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, M.; Zhao, T.; Feng, M.; Chen, H.; Zhuang, M.; Lin, L. Bioactive profiles, antioxidant activities, nitrite scavenging capacities and protective effects on H2O2-injured PC12 cells of Glycyrrhiza glabra L. leaf and root extracts. Molecules 2014, 19, 9101–9113. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Giofrè, S.V.; Tomaino, A.; Raciti, R.; Saija, A.; Cristani, M.; Romeo, R.; Siracusa, L.; Ruberto, G. Selective COX-2 inhibitory properties of dihydrostilbenes from liquorice leaves—In Vitro assays and structure/activity relationship study. Nat. Prod. Commun. 2014, 9, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Biondi, D.M.; Rocco, C.; Ruberto, G. New dihydrostilbene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity. J. Nat. Prod. 2003, 66, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Biondi, D.M.; Rocco, C.; Ruberto, G. Dihydrostilbene derivatives from Glycyrrhiza glabra leaves. J. Nat. Prod. 2005, 68, 1099–1102. [Google Scholar] [CrossRef]
- Aiello, F.; Armentano, B.; Polerà, N.; Carullo, G.; Loizzo, M.R.; Bonesi, M.; Cappello, M.S.; Capobianco, L.; Tundis, R. From Vegetable Waste to New Agents for Potential Health Applications: Antioxidant Properties and Effects of Extracts, Fractions and Pinocembrin from Glycyrrhiza glabra L. Aerial Parts on Viability of Fuve Human Cancer Cell Lines. J. Agric. Food Chem. 2017, 66, 7944–7954. [Google Scholar] [CrossRef]
- Hayashi, H.; Hiraoka, N.; Ikeshiro, Y.; Yamamoto, H. Organ specific localization of flavonoids in Glycyrrhiza glabra L. Plant Sci. 1996, 116, 233–238. [Google Scholar] [CrossRef]
- Tundis, R.; Frattaruolo, L.; Carullo, G.; Armentano, B.; Badolato, M.; Loizzo, M.R.; Aiello, F.; Cappello, A.R. An ancient remedial repurposing: Synthesis of new Pinocembrin fatty acid acyl derivatives as potential antimicrobial/anti-inflammatory agents. Nat. Prod. Res. 2019, 33, 162–168. [Google Scholar] [CrossRef]
- Governa, P.; Carullo, G.; Biagi, M.; Rago, V.; Aiello, F. Evaluation of the In Vitro Wound-Healing Activity of Calabrian Honeys. Antioxidants 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Millimouno, F.M.; Ali Eltayb, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A Novel Natural Compound with Versatile Pharmacological and Biological Activities. BioMed Res. Int. 2013, 2013, 379850. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Saija, A.; Cristani, M.; Cimino, F.; D’Arrigo, M.; Trombetta, D.; Rao, F.; Ruberto, G. Phytocomplexes from liquorice (Glycyrrhiza glabra L.) leaves—Chemical characterization and evaluation of their antioxidant, anti-genotoxic and anti-inflammatory activity. Fitoterapia 2011, 82, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Li, Y.; Zheng, Y.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.; Chen, X.; Ji, H.; Zhang, Y.; Liu, A. Pinocembrin-Lecithin Complex: Characterization, Solubilization, and Antioxidant Activities. Biomolecules 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Plastina, P.; Apriantini, A.; Meijerink, J.; Witkamp, R.; Gabriele, B.; Fazio, A. In Vitro Anti-Inflammatory and Radical Scavenging Properties of Chinotto (Citrus myrtifolia Raf.) Essential Oils. Nutrients 2018, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Giorgi, G.; Spizzirri, U.G.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Technol. 2019, 54, 1313–1320. [Google Scholar] [CrossRef]
- Bonesi, M.; Brindisi, M.; Armentano, B.; Curcio, R.; Sicari, V.; Loizzo, M.R.; Cappello, M.S.; Bedini, G.; Peruzzi, L.; Tundis, R. Exploring the anti-proliferative, pro-apoptotic, and antioxidant properties of Santolina corsica Jord. & Fourr.(Asteraceae). Biomed. Pharmacother. 2018, 107, 967–978. [Google Scholar]
- Carrisi, C.; Madeo, M.; Morciano, P.; Dolce, V.; Cenci, G.; Cappello, A.R.; Mazzeo, G.; Iacopetta, D.; Capobianco, L. Identification of the Drosophila melanogaster mitochondrial citrate carrier: Bacterial expression, reconstitution, functional characterization and developmental distribution. J. Biochem. 2008, 144, 389–392. [Google Scholar] [CrossRef]
- Li, Y.; Cappello, A.R.; Muto, L.; Martello, E.; Madeo, M.; Curcio, R.; Lunetti, P.; Raho, S.; Zaffino, F.; Frattaruolo, L.; et al. Functional characterization of the partially purified Sac1p independent adenine nucleotide transport system (ANTS) from yeast endoplasmic reticulum. J. Biochem. 2018, 164, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.A.; Santi, M.D.; Agnese, A.M.; Cabrera, J.L.; Ortega, M.G. Flavanoids from Dalea elegans: Chemical reassignment and determination of kinetics parameters related to their anti-tyrosinase activity. Phytochem. Lett. 2014, 10, 260–267. [Google Scholar] [CrossRef]
- Fukui, H.; Goto, K.; Tabata, M. Two Antimicrobial Flavanones from the Leaves of Glycyrrhiza glabra. Chem. Pharm. Bull. 1988, 36, 4174–4176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pathania, A.S.; Nalli, Y.K.; Malik, F.A.; Vishwakarma, R.A.; Ali, A. Synthesis of new O-alkyl and alkyne-azide cycloaddition derivatives of 4′-methoxy Licoflavanone: A distinct prenylated flavonoids depicting potent cytotoxic activity. Med. Chem. Res. 2015, 24, 669–683. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Solco, A.; Wu, L.; Wurtele, E.S.; Kohut, M.L.; Murphy, P.A.; Cunnick, J.E. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. J. Ethnopharmacol. 2009, 122, 76–85. [Google Scholar] [Green Version]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Sukumaran, V.; Park, S.C. Pinocembrin attenuates lipopolysaccharide-induced inflammatory responses in Labeo rohita macrophages via the suppression of the NF-κB signalling pathway. Fish Shellfish Immunol. 2016, 56, 459–466. [Google Scholar] [CrossRef]
- Pannu, A.; Goyal, R.K.; Ojha, S.; Nandave, M. Naringenin: A Promising Flavonoid for Herbal Treatment of Rheumatoid Arthritis and Associated Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Academic Press: Cambridge, MA, USA, 2019; pp. 343–354. [Google Scholar]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 23697. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Xiao, J. Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Vienna, Austria, 2018; pp. 45–67. [Google Scholar]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. 2019. [Google Scholar] [CrossRef] [PubMed]






| Primer Name | Sequence (5′-3′) |
|---|---|
| iNOS-Fw | CGAAACGCTTCACTTCCAA |
| iNOS-Rv | TGAGCCTATATTGCTGTGGCT |
| COX2-Fw | AACCGCATTGCCTCTGAAT |
| COX2-Rv | CATGTTCCAGGAGGATGGAG |
| TNFa-Fw | CAGGCGGTGCCTATGTCTC |
| TNFa –Rv | CGATCACCCCGAAGTTCAGTAG |
| IL1b-Fw | GAAATGCCACCTTTTGACAGTG |
| IL1b-Rv | TGGATGCTCTCATCAGGACAG |
| IL6-Fw | CTGCAAGAGACTTCCATCCAG |
| IL6-Rv | AGTGGTATAGACAGGTCTGTTGG |
| GAPDH-Fw | ACCACAGTCCATGCCATCAC |
| GAPDH-Rv | TCCACCACCCTGTTGCTGTA |
| IC50 ± SD (µg/mL) | IC50 ± SD (µM) | |||||
|---|---|---|---|---|---|---|
| Extract A | Extract B | Extract C | M2 | M3 | M4 | |
| DPPH (IC50) | 13.49 ± 1.91 | 18.05 ± 4.3 | 15.5 ± 2.5 | n.c. | n.c. | n.c. |
| ABTS (IC50) | 6.76 ± 0.78 | 6.1 ± 1.04 | 5.88 ± 0.83 | 166.3 ± 47.7 | n.c. | 59.55 ± 21.9 |
| Extract A | Extract B | Extract C | |
|---|---|---|---|
| Total phenolic content (mg Gallic Acid equivalents/g extract) | 154.03 ± 10.03 | 190.37 ± 13.95 | 165.63 ± 9.21 |
| IC50 ± SD (µg/mL) | IC50 ± SD (µM) | ||||
|---|---|---|---|---|---|
| Extract A | Extract B | Extract C | M2 | M3 | M4 |
| 17.72 ± 3.79 | 11.73 ± 2.09 | 16.11 ± 1.5 | 60.49 ± 26.78 | 206.5 ± 44.89 | 37.68 ± 6.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway. Antioxidants 2019, 8, 186. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8060186
Frattaruolo L, Carullo G, Brindisi M, Mazzotta S, Bellissimo L, Rago V, Curcio R, Dolce V, Aiello F, Cappello AR. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway. Antioxidants. 2019; 8(6):186. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8060186
Chicago/Turabian StyleFrattaruolo, Luca, Gabriele Carullo, Matteo Brindisi, Sarah Mazzotta, Luca Bellissimo, Vittoria Rago, Rosita Curcio, Vincenza Dolce, Francesca Aiello, and Anna Rita Cappello. 2019. "Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway" Antioxidants 8, no. 6: 186. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8060186