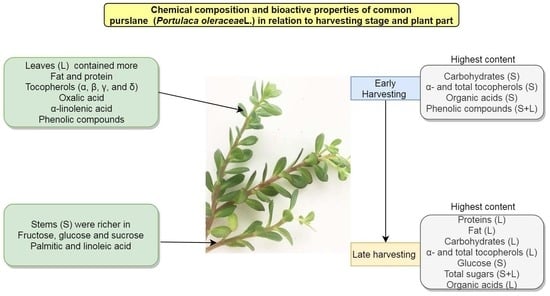
Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca oleracea L.) in Relation to Harvesting Stage and Plant Part
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Chemical Analyses
2.2.1. Standards and Reagents
2.2.2. Nutritional Compounds and Energetic Value
2.2.3. Tocopherols
2.2.4. Free Sugars
2.2.5. Organic Acids
2.2.6. Fatty Acids
2.2.7. Phenolic Compounds and Oleracein Derivatives
2.3. Cytotoxicity
2.3.1. Cytotoxicity in Non-Tumor Liver Cell Primary Culture
2.3.2. Cytotoxicity in Human Tumor Cell Lines
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Phytochemical Composition and Bioactive Compounds of Common Purslane (Portulaca oleracea L.) as Affected by Crop Management Practices. Trends Food Sci. Technol. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Nebel, S.; Heinrich, M. Ta chórta: A Comparative Ethnobotanical-Linguistic Study of Wild Food Plants in a Graecanic Area in Calabria, Southern Italy. Econ. Bot. 2009, 63, 78–92. [Google Scholar] [CrossRef]
- Mitich, L.W. Common Purslane (Portulaca oleracea). Weed Technol. 1997, 11, 394–397. [Google Scholar] [CrossRef]
- Oliveira, I.; Valentão, P.; Lopes, R.; Andrade, P.B.; Bento, A.; Pereira, J.A. Phytochemical Characterization and Radical Scavenging Activity of Portulaca oleraceae L. Leaves and Stems. Microchem. J. 2009, 92, 129–134. [Google Scholar] [CrossRef]
- Mohamed, A.; Hussein, A. Chemical Composition of Purslane (Portulaca oleracea). Plant Foods Hum. Nutr. 1994, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Howe, P.; Zhou, Y.F.; Xu, Z.Q.; Hocart, C.; Zhang, R. Fatty Acids and β-Carotene in Australian Purslane (Portulaca oleracea) Varieties. J. Chromatogr. A 2000, 893, 207–213. [Google Scholar] [CrossRef]
- Szalai, G.; Dai, N.; Danin, A.; Dudai, N.; Barazani, O. Effect of Nitrogen Source in the Fertilizing Solution on Nutritional Quality of Three Members of the Portulaca oleracea Aggregate. J. Sci. Food Agric. 2010, 90, 2039–2045. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.R.; Tundis, R.; Mincione, A.; Pellicanò, T.M. Portulaca oleracea L. (Purslane) Extracts Display Antioxidant and Hypoglycaemic Effects. J. Appl. Bot. Food Qual. 2018, 91, 39–46. [Google Scholar] [CrossRef]
- Naser Aldeen, M.; Mansour, R.; AlJoubbeh, M. The Effect of Food Additives and Cooking on the Antioxidant Properties of Purslane. Nutr. Food Sci. 2019. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and Metabolic changes of Purslane (Portulaca oleracea L.) in Response to Drought, Heat, and Combined Stresses. Front. Plant Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Rahdari, P.; Tavakoli, S.; Hosseini, S.M. Studying of Salinity Stress Effect on Germination, Proline, Sugar, Protein, Lipid and Chlorophyll Content in Purslane (Portulaca oleracea L.) Leaves. J. Sress Physiol. Biochem. 2012, 8, 182–193. [Google Scholar]
- Uddin, K.; Juraimi, A.S.; Anwar, F.; Hossain, M.A.; Alam, M.A. Effect of Salinity on Proximate Mineral Composition of Purslane (Portulacaoleracea L.). Aust. J. Crop Sci. 2012, 6, 1732–1736. [Google Scholar]
- Teixeira, M.; Carvalho, I.S. Effects of Salt Stress on Purslane (Portulaca oleracea) Nutrition. Ann. Appl. Biol. 2009, 154, 77–86. [Google Scholar] [CrossRef]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Alam, M.Z. Effects of Salinity and Salinity-Induced Augmented Bioactive Compounds in Purslane (Portulaca oleracea L.) for Possible Economical Use. Food Chem. 2015, 169, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Karkanis, A.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R.; Ntatsi, G.; Petrotos, K.; Lykas, C.; Khah, E. Chemical Composition and Yield of Six Genotypes of Common Purslane (Portulaca oleracea L.): An Alternative Source of Omega-3 Fatty Acids. Plant Foods Hum. Nutr. 2015, 70, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.Y.; Abdul Hamid, A.; Aslani, F.; Hasan, M.M.; Mohd Zainudin, M.A.; Uddin, M.K. Evaluation of Antioxidant Compounds, Antioxidant Activities, and Mineral Composition of 13 Collected Purslane (Portulaca oleracea L.) Accessions. Biomed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Ezekwe, M.O.; Omara-Alwala, T.R.; Membrahtu, T. Nutritive Characterization of Purslane Accessions as Influenced by Planting Date. Plant Foods Hum. Nutr. 1999, 54, 183–191. [Google Scholar] [CrossRef]
- Montoya-García, C.O.; Volke-Haller, V.H.; Trinidad-Santos, A.; Villanueva-Verduzco, C. Change in the Contents of Fatty Acids and Antioxidant Capacity of Purslane in Relation to Fertilization. Sci. Hortic. (Amst.) 2018, 234, 152–159. [Google Scholar] [CrossRef]
- Fontana, E.; Hoeberechts, J.; Nicola, S.; Cros, V.; Palmegiano, G.B.; Peiretti, P.G. Nitrogen Concentration and Nitrate Ammonium Ratio Affect Yield and Change the Oxalic Acid Concentration and Fatty Acid Profile of Purslane (Portulaca oleracea L.) Grown in a Soilless Culture System. J. Sci. Food Agric. 2006, 86, 2417–2424. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Ali, M.E.; Ismail, M.R. Evaluation of Antioxidant Properties and Mineral Composition of Purslane (Portulaca oleracea L.) at Different Growth Stages. Int. J. Mol. Sci. 2012, 13, 10257–10267. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G., Eds.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Composition of Wild and Commercial Achillea millefolium L. and Bioactivity of the Methanolic Extract, Infusion and Decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and Chemical Characterization in Hydrophilic and Lipophilic Compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Obodai, M.; Mensah, D.L.N.; Fernandes, Â.; Kortei, N.K.; Dzomeku, M.; Teegarden, M.; Schwartz, S.J.; Barros, L.; Prempeh, J.; Takli, R.K.; et al. Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana. Molecules 2017, 22, 196. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic Profile and Antioxidant Activity of Coleostephus myconis (L.) Rchb.f.: An Underexploited and Highly Disseminated Species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.-J.R.P. Anti-Hepatocellular Carcinoma Activity Using Human HepG2 Cells and Hepatotoxicity of 6-Substituted Methyl 3-Aminothieno[3,2-b]Pyridine-2-Carboxylate Derivatives: In Vitro Evaluation, Cell Cycle Analysis and QSAR Studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Ezeabara, C.A.; Faith, I.C.; Ilodibia, C.V.; Aziagba, B.O.; Okanume, O.E.; Ike, M.E. Comparative Determination of Phytochemical, Proximate and Mineral Compositions in Various Parts of Portulaca oleracea L. J. Plant Sci. 2014, 2, 294–298. [Google Scholar] [CrossRef]
- Tucker, J.M.; Townsend, D.M. Alpha-Tocopherol: Roles in Prevention and Therapy of Human Disease. Biomed. Pharmacother. 2005, 59, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. CRC. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Jin, R.; Shi, H.; Han, C.; Zhong, B.; Wang, Q.; Chan, Z. Physiological Changes of Purslane (Portulaca oleracea L.) after Progressive Drought Stress and Rehydration. Sci. Hortic. (Amsterdam). 2015, 194, 215–221. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Rodríguez-García, I. Lipids Classes, Fatty Acids and Carotenes of the Leaves of Six Edible Wild Plants. Eur. Food Res. Technol. 1999, 209, 313–316. [Google Scholar] [CrossRef]
- Guil, J.L.; Torija, M.E.; Giménez, J.J.; Rodriguez, I. Identification of Fatty Acids in Edible Wild Plants by Gas Chromatography. J. Chromatogr. A 1996, 719, 229–235. [Google Scholar] [CrossRef]
- Xing, J.; Yang, Z.; Lv, B.; Xiang, L. Rapid Screening for Cyclo-Dopa and Diketopiperazine Alkaloids in Crude Extracts of Portulaca oleracea L. Using Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, C.; Xiang, L.; Zheng, Y. Phenolic Alkaloids as a New Class of Antioxidants in Portulaca oleracea. Phyther. Res. 2009, 23, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xing, D.; Wang, W.; Wang, R.; Ding, Y.; Du, L. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Shakour, Z.T.A. Metabolomics Driven Analysis of 11 Portulaca Leaf Taxa as Analysed via UPLC-ESI-MS/MS and Chemometrics. Phytochemistry 2019, 161, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Quah, E.P.L. Antioxidant Properties of Different Cultivars of Portulaca oleracea. Food Chem. 2007, 103, 734–740. [Google Scholar] [CrossRef]
- Gallo, M.; Conte, E.; Naviglio, D. Analysis and Comparison of the Antioxidant Component of Portulaca oleracea Leaves Obtained by Different Solid-Liquid Extraction Techniques. Antioxidants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Mortazavi, P.; Moghadam, J.Z.; Mardani, P.M. Hepatoprotective Effects of Portulaca oleracea Extract against CCl 4 -Induced Damage in Rats. Pharm. Biol. 2015, 53, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Abdalla, H.; Soad Mohamed, A.G. In vivo Hepato-protective Properties of Purslane Extracts on Paracetamol-Induced Liver Damage. Mal. J. Nutr. 2010, 16, 161–170. [Google Scholar]
- Yen, G.C.; Chen, H.Y.; Peng, H.H. Evaluation of the Cytotoxicity, Mutagenicity and Antimutagenicity of Emerging Edible Plants. Food Chem. Toxicol. 2001, 39, 1045–1053. [Google Scholar] [CrossRef]
- Choi, B.-D.; Ryeom, K. Screening of Antitumor Activity from the Crude Drugs in Korea. Korean J. Pharmacogn. 2000, 31, 16–22. [Google Scholar]
- Zhao, R.; Gao, X.; Cai, Y.; Shao, X.; Jia, G.; Huang, Y.; Qin, X.; Wang, J.; Zheng, X. Antitumor Activity of Portulaca oleracea L. Polysaccharides against Cervical Carcinoma in Vitro and in Vivo. Carbohydr. Polym. 2013, 96, 376–383. [Google Scholar] [CrossRef] [PubMed]

| Harvest Stage (DAS) * | Plant Part | Moisture (%) | Fat | Proteins | Ash | Carbohydrates | Energy |
|---|---|---|---|---|---|---|---|
| 29 | Stems | 88.49 ± 0.41c | 0.111 ± 0.002a | 1.31 ± 0.01b | 2.48 ± 0.09a | 7.6 ± 0.1a | 47 ± 9a |
| Leaves | 91.00 ± 0.49a | 0.157 ± 0.001b | 1.57 ± 0.02c | 2.14 ± 0.05b | 5.13 ± 0.02c | 43.2 ± 0.1c | |
| 43 | Stems | 91.97 ± 0.08a | 0.091 ± 0.001b | 0.76 ± 0.01c | 1.78 ± 0.03b | 5.40 ± 0.02b | 35.54 ± 0.03c |
| Leaves | 90.81 ± 0.16a | 0.148 ± 0.002b | 1.91 ± 0.01b | 1.89 ± 0.05c | 5.25 ± 0.03b | 45.70 ± 0.02b | |
| 52 | Stems | 91.31 ± 0.1b | 0.111 ± 0.003a | 1.44 ± 0.01a | 1.74 ± 0.04b | 5.39 ± 0.03b | 41.52 ± 0.02b |
| Leaves | 88.16 ± 0.41b | 0.230 ± 0.001a | 2.96 ± 0.04a | 2.40 ± 0.06a | 6.2 ± 0.1a | 61.3 ± 0.1a | |
| Student’s t | Plant Part ** | <0.001 | <0.001 | <0.01 | <0.01 | <0.01 | <0.01 |
| Harvest Stage (DAS) * | Plant Part | α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total Tocopherols |
| 29 | Stems | 26.0 ± 0.2a | 3.4 ± 0.3a | 14.4 ± 0.1a | 0.99 ± 0.03 | 44.7 ± 0.5a |
| Leaves | 215 ± 4b | 14.0 ± 0.7b | 140.7 ± 0.1a | 9.6 ± 0.5b | 380 ± 4b | |
| 43 | Stems | 19.3 ± 0.5b | 3.6 ± 0.6a | 8.3 ± 0.1b | nd | 31 ± 1b |
| Leaves | 197 ± 3c | 12.4 ± 0.2b | 87.7 ± 0.2c | 5.1 ± 0.2c | 302 ± 2c | |
| 52 | Stems | 10.4 ± 0.2c | 1.8 ± 0.1b | 5.2 ± 0.2c | nd | 17.4 ± 0.6c |
| Leaves | 327 ± 3a | 44 ± 2a | 97 ± 8b | 13.5 ± 0.5a | 481 ± 9a | |
| Student’s t | Plant part | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Harvest Stage (DAS) * | Plant Part | Fructose | Glucose | Sucrose | Trehalose | Total Sugars |
| 29 | Stems | 0.308 ± 0.006c | 0.358 ± 0.001c | 0.135 ± 0.001a | 0.024 ± 0.001a | 0.830 ± 0.006c |
| Leaves | 0.11 ± 0.01b | 0.041 ± 0.002c | nd | 0.012 ± 0.001c | 0.160 ± 0.007b | |
| 43 | Stems | 0.44 ± 0.02a | 0.53 ± 0.02b | 0.051 ± 0.001c | 0.012 ± 0.001b | 1.03 ± 0.04b |
| Leaves | 0.183 ± 0.007a | 0.113 ± 0.002a | 0.009 ± 0.001a | 0.026 ± 0.001b | 0.330 ± 0.009a | |
| 52 | Stems | 0.39 ± 0.01b | 0.74 ± 0.02a | 0.118 ± 0.003b | 0.022 ± 0.001a | 1.28 ± 0.04a |
| Leaves | 0.179 ± 0.007a | 0.100 ± 0.001b | 0.014 ± 0.001a | 0.041 ± 0.001a | 0.330 ± 0.008a | |
| Student’s t | Plant part ** | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Harvest Stage (DAS) * | Plant Part | Oxalic Acid | Quinic Acid | Malic Acid | Citric Acid | Total Organic Acids |
|---|---|---|---|---|---|---|
| 29 | Stems | 7.70 ± 0.07a | 6.36 ± 0.04a | 6.39 ± 0.07b | 5.00 ± 0.03a | 25.5 ± 0.2a |
| Leaves | 6.2 ± 0.1b | 6.82 ± 0.01c | 3.00 ± 0.03a | 3.26 ± 0.01a | 19.2 ± 0.1b | |
| 43 | Stems | 4.77 ± 0.01c | 1.31 ± 0.01c | 6.56 ± 0.07a | 1.57 ± 0.04c ¥ | 14.22 ± 0.04c |
| Leaves | 5.7 ± 0.1c | 8.4 ± 0.2b | 1.90 ± 0.04b | 1.53 ± 0.02b ¥ | 17.6 ± 0.1c | |
| 52 | Stems | 7.16 ± 0.02b | 3.57 ± 0.04b | 5.38 ± 0.06c | 2.78 ± 0.01b | 18.89 ± 0.04b |
| Leaves | 8.6 ± 0.2a | 16.8 ± 0.5a | 1.67 ± 0.01c | 3.24 ± 0.03a | 30.3 ± 0.2a | |
| 80 | Seeds | 0.470 ± 0.005 | nd | tr | tr | 0.470 ± 0.005 |
| Student’s t | Plant part ** | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Fatty Acids | 29 DAS | 43 DAS | 52 DAS | Student’s t | |||
|---|---|---|---|---|---|---|---|
| Stems | Leaves | Stems | Leaves | Stems | Leaves | Plant Parts ** | |
| C6:0 | 0.543 ± 0.001a | 0.024 ± 0.001c | 0.30 ± 0.03b | 0.067 ± 0.001b | 0.280 ± 0.006c | 0.220 ± 0.001a | <0.001 |
| C8:0 | 0.098 ± 0.003c | 0.032 ± 0.003c | 0.12 ± 0.01a | 0.039 ± 0.001b | 0.115 ± 0.008b | 0.095 ± 0.007a | <0.001 |
| C10:0 | 0.100 ± 0.003a | 0.052 ± 0.001b | 0.091 ± 0.004b | 0.051 ± 0.001b | 0.094 ± 0.006b | 0.125 ± 0.007a | <0.001 |
| C12:0 | 0.93 ± 0.03a | 0.81 ± 0.02c | 0.62 ± 0.04b | 0.867 ± 0.001b | 0.640 ± 0.006b | 1.37 ± 0.04a | <0.001 |
| C14:0 | 1.42 ± 0.02a | 0.736 ± 0.002c | 0.97 ± 0.02b | 0.77 ± 0.01b | 0.85 ± 0.02c | 1.24 ± 0.01a | <0.001 |
| C15:0 | 0.45 ± 0.01b | 0.49 ± 0.01b | 0.472 ± 0.008b | 0.420 ± 0.003c | 0.62 ± 0.02a | 0.75 ± 0.01a | <0.001 |
| C16:0 | 21.8 ± 0.2a | 9.8 ± 0.1c | 21.0 ± 0.1b | 10.83 ± 0.01b | 20.2 ± 0.2c | 12.39 ± 0.03a | <0.001 |
| C16:1 | 0.236 ± 0.009c | 0.52 ± 0.01b | 0.36 ± 0.01b | 0.48 ± 0.01c | 0.401 ± 0.009a | 0.730 ± 0.001a | <0.001 |
| C17:0 | 0.54 ± 0.03b | 0.15 ± 0.01c | 0.48 ± 0.02c | 0.159 ± 0.005b | 0.816 ± 0.007a | 0.265 ± 0.007a | <0.001 |
| C18:0 | 4.9 ± 0.1a | 2.52 ± 0.05c | 4.55 ± 0.05c | 2.72 ± 0.01b | 4.83 ± 0.07b | 3.89 ± 0.06a | <0.001 |
| C18:1n9c+t | 9.55 ± 0.04b | 5.29 ± 0.05b | 11.62 ± 0.01a | 4.65 ± 0.04c | 6.83 ± 0.14c | 6.4 ± 0.1a | <0.001 |
| C18:2n6c | 23.02 ± 0.02c | 11.40 ± 0.08c | 27.11 ± 0.02a | 11.63 ± 0.02b | 25.4 ± 0.2b | 14.81 ± 0.02a | <0.001 |
| C18:3n3 | 17.31 ± 0.04a | 54.92 ± 0.08a | 15.03 ± 0.07b | 54.34 ± 0.03a | 11.64 ± 0.01c | 35.4 ± 0.1b | <0.001 |
| C20:0 | 1.86 ± 0.06c | 1.79 ± 0.01b | 1.99 ± 0.03c | 1.80 ± 0.01b | 2.2 ± 0.1a | 2.95 ± 0.03a | <0.001 |
| C20:1CIS-11 | 0.40 ± 0.01a | 0.08 ± 0.01c | 0.258 ± 0.006b | 0.11 ± 0.01b | 0.146 ± 0.001c ¥ | 0.140 ± 0.001a ¥ | <0.001 |
| C20:3n3+C21:0 | 0.67 ± 0.01a | 0.155 ± 0.004c | 0.63 ± 0.04c | 0.195 ± 0.004b | 0.646 ± 0.002b | 0.32 ± 0.02a | <0.001 |
| C20:5n3 | 0.17 ± 0.01a | 0.051 ± 0.003a | 0.17 ± 0.01a | 0.042 ± 0.001b | 0.146 ± 0.003b | 0.040 ± 0.001b | <0.001 |
| C22:0 | 11.0 ± 0.2b | 9.0 ± 0.3b | 9.6 ± 0.2c | 8.62 ± 0.09c | 15.40 ± 0.23a | 15.0 ± 0.2a | <0.001 |
| C23:0 | 0.44 ± 0.02c | 0.20 ± 0.01b | 0.57 ± 0.01b | 0.15 ± 0.01c | 0.77 ± 0.02a | 0.31 ± 0.01a | <0.001 |
| C24:0 | 4.5 ± 0.1b | 2.04 ± 0.08b | 4.1 ± 0.2c | 2.05 ± 0.01b | 7.97 ± 0.02a | 3.61 ± 0.04a | <0.001 |
| Total SFA (% of total FA) | 48.65 ± 0.02b | 27.58 ± 0.06c | 44.82 ± 0.01c | 28.5 ± 0.1b | 54.8 ± 0.1a | 42.2 ± 0.3a | <0.001 |
| Total MUFA (% of total FA) | 10.18 ± 0.04b | 5.89 ± 0.05b | 12.24 ± 0.02a | 5.25 ± 0.06c | 7.38 ± 0.13c ¥ | 7.3 ± 0.1a ¥ | <0.001 |
| Total PUFA (% of total FA) | 41.17 ± 0.02b | 66.53 ± 0.01a | 42.94 ± 0.02a | 66.21 ± 0.04b | 37.81 ± 0.25c | 50.5 ± 0.2c | <0.001 |
| PUFA/SFA | 0.864 ± 0.001b | 2.412 ± 0.003a | 0.958 ± 0.001a | 2.319 ± 0.007b | 0.690 ± 0.004c | 1.196 ± 0.009c | <0.001 |
| n6/n3 | 1.269 ± 0.005c | 0.207 ± 0.001c | 1.71 ± 0.01b | 0.213 ± 0.001b | 2.04 ± 0.01a | 0.414 ± 0.002a | <0.001 |
| Peak | Rt (min) | λmax (nm) | [M−H]− (m/z) | MS2 (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 6.82 | 345 | 664 | 502(100), 340(20), 296(5), 194(3) | Oleracein C |
| 2 | 8.77 | 323 | 179 | 161(100), 143(63), 119(40) | Caffeic acid |
| 3 | 9.29 | 329 | 385 | 223 (100) | Sinapic acid hexoside |
| 4 | 11.89 | 318 | - | 179(100), 161(55), 143(31), 119(18) | Caffeic acid derivative |
| 5 | 15.54 | 338 | 502 | 340(100), 296(5), 194(3), 145(3) | Oleracein A |
| Peak | Phenolic Compound | 29 | 43 | 52 | Plant Parts ** | |||
|---|---|---|---|---|---|---|---|---|
| Stems | Leaves | Stems | Leaves | Stems | Leaves | |||
| 1 | Oleracein C A | 15.2 ± 0.5a | 143 ± 5a | 6.7 ± 0.1b | 21.2 ± 0.3c | 3.34 ± 0.07c | 102 ± 2b | <0.01 |
| 2 | Caffeic acid B | 0.44 ± 0.02a | nd | 0.45 ± 0.01a | nd | tr | nd | <0.01 |
| 3 | Sinapic acid hexoside C | 4.2 ± 0.1a | 22.1 ± 0.7a | 4.3 ± 0.2a | nd | 1.37 ± 0.03b | nd | <0.01 |
| 4 | Cafferic acid derivative | nd | nd | nd | nd | tr | nd | - |
| 5 | Oleracein A A | nd | 103 ± 2a | 0.75 ± 0.01a | 8.2 ± 0.1c | 0.28 ± 0.02b | 34.9 ± 0.8b | <0.01 |
| TPCOD | 19.8 ± 0.4a | 268 ± 6a | 12.2 ± 0.1b | 29.3 ± 0.4c | 4.99 ± 0.08c | 137 ± 3b | <0.01 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca oleracea L.) in Relation to Harvesting Stage and Plant Part. Antioxidants 2019, 8, 293. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8080293
Petropoulos SA, Fernandes Â, Dias MI, Vasilakoglou IB, Petrotos K, Barros L, Ferreira ICFR. Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca oleracea L.) in Relation to Harvesting Stage and Plant Part. Antioxidants. 2019; 8(8):293. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8080293
Chicago/Turabian StylePetropoulos, Spyridon A., Ângela Fernandes, Maria Inês Dias, Ioannis B. Vasilakoglou, Konstantinos Petrotos, Lillian Barros, and Isabel C. F. R. Ferreira. 2019. "Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca oleracea L.) in Relation to Harvesting Stage and Plant Part" Antioxidants 8, no. 8: 293. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox8080293