Transfer and Enzyme-Mediated Metabolism of Oxidized Phosphatidylcholine and Lysophosphatidylcholine between Low- and High-Density Lipoproteins
Abstract
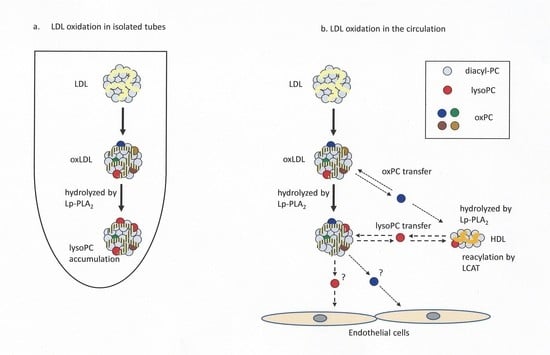
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Human Lipoproteins
2.3. Preparation of 16:0 d13-lysoPC from 16:0-16:0 d13-PC
2.4. Preparation of d13-PGPC
2.5. LDL Labeling with 16:0 d13-lysoPC
2.6. LDL Labeling with d13-PGPC
2.7. Inhibition of Lp-PLA2 or LCAT Activity in HDL
2.8. Assessment of lysoPC Transfer and PC Generation
2.9. Assessment of LCAT Function
2.10. Assessment of PGPC Metabolism
2.11. LC-MS/MS Analysis of Molecular Species d13-Labeled PCs
2.12. Statistical Analysis
3. Results
3.1. Transfer and Metabolism of d13-lysoPC
3.2. Transfer and Metabolism of d13-PGPC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherosclerosis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 2008, 283, 15527–15531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itabe, H.; Kato, R.; Sasabe, N.; Obama, T.; Yamamoto, M. Significance of oxidized low-density lipoprotein in body fluids as a marker related to diseased conditions. Curr. Med. Chem. 2019, 26, 1576–1593. [Google Scholar] [CrossRef]
- Itabe, H.; Takeshima, E.; Iwasaki, H.; Kimura, J.; Yoshida, Y.; Imanaka, T.; Takano, T. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholine and polypeptides. J. Biol. Chem. 1994, 269, 15274–15279. [Google Scholar] [PubMed]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otuska, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Oxidized low density lipoprotein relates to plaque destabilization in human coronary atherosclerotic lesions. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.E.; Khoo, J.C.; Miller, E.R.; Parthasarathy, S.; Palinski, W.; Witztum, J.L. Macrophage-derived foam cells freshly isolated from rabbit atherosclerotic lesions degrade modified lipoproteins, promote oxidation of low-density lipoproteins, and contain oxidation-specific lipid-protein adducts. J. Clin. Investig. 1991, 87, 90–99. [Google Scholar] [CrossRef]
- Tsimikas, S.; Bergmark, C.; Beyer, R.W.; Patel, R.; Pattison, J.; Miller, E.; Juliano, J.; Witztum, J.L. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J. Am. Coll. Cardiol. 2003, 41, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Itabe, H.; Ueda, M. Measurement of plasma oxidized low-density lipoprotein and its clinical implications. J. Atheroscler. Thromb. 2007, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; DiDonato, J.A.; Levison, B.S.; Schmitt, D.; Li, L.; Wu, Y.; Buffa, J.; Kim, T.; Gerstenecker, G.S.; Gu, X.; et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014, 20, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Sawada, N.; Obama, T.; Koba, S.; Takaki, T.; Iwamoto, S.; Aiuchi, T.; Kato, R.; Kikuchi, M.; Hamazaki, Y.; Itabe, H. Circulating oxidized LDL increased in patients with acute myocardial infarction is accompanied by heavily modified HDL. J. Lipid Res. 2020, 61, 816–829. [Google Scholar] [CrossRef] [Green Version]
- Itabe, H. Oxidative modification of LDL: Its pathological role in atherosclerosis. Clin. Rev. Allegy. Immunol. 2009, 37, 4–11. [Google Scholar] [CrossRef]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL diversity, patterns of recognition, and pathophysiology. Antioxid. Redox Signal. 2010, 13, 39–75. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Ashraf, M.Z.; Kar, N.S.; Lin, D.; Sayre, L.M.; Podrez, E.A. Structural basis for the recognition of oxidized phospholipids in oxidized low density lipoproteins by class B scavenger receptor CD36 and SR-BI. J. Biol. Chem. 2010, 285, 4447–4454. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, I.; Edsfeldt, A.; Ko, N.Y.; Grufman, H.; Berg, K.; Björkbacka, H.; Nitulescu, M.; Persson, A.; Nilsson, M.; Prehn, C.; et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb Vac. Biol. 2012, 32, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Chisolm, G.M., III; Chai, Y.-C. Regulation of cell growth by oxidized LDL. Free Rad. Biol. Med. 2000, 28, 1697–1707. [Google Scholar] [CrossRef]
- Davis, B.; Koster, G.; Douet, L.J.; Scigelova, M.; Woffendin, G.; Ward, J.M.; Smith, A.; Humphries, J.; Burnand, K.G.; Macphee, C.H.; et al. Electrospray ionization mass spectrometry identifies substrates and products of lipoprotein-associated phospholipase A2 in oxidized human low density lipoprotein. J. Biol. Chem. 2008, 283, 6428–6437. [Google Scholar] [CrossRef] [Green Version]
- Sasabe, N.; Keyamura, Y.; Obama, T.; Inoue, N.; Masuko, Y.; Igarashi, Y.; Aiuchi, T.; Kato, R.; Yamaguchi, T.; Kuwata, H.; et al. Time course-changes in phosphatidylcholine profile during oxidative modification of low-density lipoprotein. Lipids Health Dis. 2014, 13, 48. [Google Scholar] [CrossRef] [Green Version]
- Rosenson, R.S.; Stafforini, D.M. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J. Lipid Res. 2012, 53, 1767–1782. [Google Scholar] [CrossRef] [Green Version]
- Itabe, H.; Yamamoto, H.; Suzuki, M.; Kawai, Y.; Nakagawa, Y.; Suzuki, A.; Imanaka, T.; Takano, T. Oxidized phosphatidylcholines that modify proteins. Analysis by monoclonal antibody against oxidized low density lipoprotein. J. Biol. Chem. 1996, 271, 33208–33217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 11–917. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.M.; Radhakrishnan, R.; Khorana, H.G. Glycerophospholipid synthesis: Improved general method and new analogs containing photoactivable groups. Proc. Natl. Acad. Sci. USA 1977, 74, 4315–4319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosaka, T.; Yamaguchi, M.; Soda, Y.; Kishimoto, T.; Tago, A.; Toyosato, M.; Mizuno, K. Spectrophotometric assay for serum platelet-activating factor acetylhydrolase activity. Clin. Chim. Acta 2000, 296, 151–161. [Google Scholar] [CrossRef]
- Goyal, J.; Wang, K.; Liu, M.; Subbaiah, P.V. Novel function of lecithin-cholesterol acyltransferase. Hydrolysis of oxidized polar phospholipids generated during lipoprotein oxidation. J. Biol. Chem. 1997, 272, 16231–16239. [Google Scholar] [CrossRef] [Green Version]
- Rasmiena, A.A.; Barlow, C.K.; Ng, T.W.; Tull, D.; Meikle, P.J. High density lipoprotein efficiently accepts surface but not internal oxidised lipids from oxidised low density lipoprotein. BBA Mol. Cell Biol. Lip. 2016, 1861, 69–77. [Google Scholar] [CrossRef]
- Shao, B. Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. BBA Mol. Cell Biol. Lip. 2012, 1821, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Sangvanich, P.; Mackness, B.; Gaskell, S.J.; Durrington, P.; Mackness, M. The effect of high-density lipoproteins on the formation of lipid/protein conjugates during in vitro oxidation of low-density lipoprotein. Biochem. Biophys. Res. Commun. 2003, 300, 501–506. [Google Scholar] [CrossRef]
- Di Donato, J.A.; Huang, Y.; Aulak, K.S.; Even-Or, O.; Gerstenecker, G.; Gogonea, V.; Wu, Y.; Fox, P.L.; Tang, W.H.W.; Plow, E.F.; et al. Function and distribution of apolipoprotein A1 in the artery wall are markedly distinct from those in plasma. Circulation 2013, 128, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, B.; Fu, X.; McDonald, T.O.; Green, P.S.; Uchida, K.; O’Brien, K.D.; Oram, J.F.; Heinecke, J.W. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J. Biol. Chem. 2005, 280, 36386–36396. [Google Scholar] [CrossRef] [Green Version]
- Subbaiah, P.V.; Liu, M.; Patlauf, F. Role of sn-2 acyl group of phosphatidylcholine in determining the positional specificity of lecithin-cholesterol acyltransferase. Biochemistry 1994, 33, 13259–13266. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Goyal, J.; Miwa, M.; Sugatani, J.; Akiyama, M.; Liu, M.; Subbaiah, P.V. Role of lecithin-cholesterol acyltransferase in the metabolism of oxidized phospholipids in plasma: Studies with platelet-activating factor-acetyl hydrolase-deficient plasma. Biochim. Biophys. Acta 1999, 1439, 95–109. [Google Scholar] [CrossRef]
- Itabe, H.; Hosoya, R.; Karasawa, K.; Jimi, S.; Saku, K.; Takebayashi, S.; Imanaka, T.; Takano, T. Metabolism of oxidized phosphatidylcholines formed in oxidized low density lipoprotein by lecithin-cholesterol acyltransferase. J. Biochem. 1999, 126, 153–161. [Google Scholar] [CrossRef]
- Massey, J.B.; Bick, D.H.; Pownall, H.J. Spontaneous transfer of monoacyl amphiphiles between lipid and protein surfaces. Biophys. J. 1997, 72, 1732–1743. [Google Scholar] [CrossRef] [Green Version]
- Podrez, E.A.; Hoppe, G.; O’Neil, J.; Hoff, H.F. Phospholipids in oxidized LDL not adducted to apoB are recognized by the CD36 scavenger receptor. Free Rad. Biol. Chem. 2003, 34, 256–264. [Google Scholar] [CrossRef]






| m/z | Molecular Species | m/z | Molecular Species |
|---|---|---|---|
| S/MUFA-d13-PC | Short chain d13-oxPC | ||
| 747.6 | 16:0-16:0 d13-PC | 623.6 | 1-palmitoyl-2-glutaroyl d13-PC |
| 745.6 | 16:0-16:1 d13-PC | 635.6 | 1-palmitoyl-2-(7-oxo-heptanoyl) d13-PC |
| 775.6 | 16:0-18:0 d13-PC | 663.6 | 1-palmitoyl-2-(9-oxo-nonanoyl) d13-PC |
| 773.6 | 16:0-18:1 d13-PC | 677.6 | 1-palmitoyl-2-(5-oxo-octenoyl) d13-PC |
| 801.6 | 16:0-20:1 d13-PC | 679.6 | 1-palmitoyl-2-azelaoyl d13-PC |
| 803.6 | 16:0-20:0 d13-PC | ||
| d13-LysoPC | |||
| PUFA-d13-PC | 509.3 | 1-palmitoyl d13-lysoPC | |
| 769.6 | 16:0-18:3 d13-PC | ||
| 771.6 | 16:0-18:2 d13-PC | ||
| 793.6 | 16:0-20:5 d13-PC | ||
| 795.6 | 16:0-20:4 d13-PC | ||
| 797.6 | 16:0-20:3 d13-PC | ||
| 799.6 | 16:0-20:2 d13-PC | ||
| 819.6 | 16:0-22:6 d13-PC | ||
| 821.6 | 16:0-22:5 d13-PC | ||
| 823.6 | 16:0-22:4 d13-PC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, N.; Obama, T.; Mizuno, M.; Fukuhara, K.; Iwamoto, S.; Aiuchi, T.; Makiyama, T.; Itabe, H. Transfer and Enzyme-Mediated Metabolism of Oxidized Phosphatidylcholine and Lysophosphatidylcholine between Low- and High-Density Lipoproteins. Antioxidants 2020, 9, 1045. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111045
Sawada N, Obama T, Mizuno M, Fukuhara K, Iwamoto S, Aiuchi T, Makiyama T, Itabe H. Transfer and Enzyme-Mediated Metabolism of Oxidized Phosphatidylcholine and Lysophosphatidylcholine between Low- and High-Density Lipoproteins. Antioxidants. 2020; 9(11):1045. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111045
Chicago/Turabian StyleSawada, Naoko, Takashi Obama, Mirei Mizuno, Kiyoshi Fukuhara, Sanju Iwamoto, Toshihiro Aiuchi, Tomohiko Makiyama, and Hiroyuki Itabe. 2020. "Transfer and Enzyme-Mediated Metabolism of Oxidized Phosphatidylcholine and Lysophosphatidylcholine between Low- and High-Density Lipoproteins" Antioxidants 9, no. 11: 1045. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111045