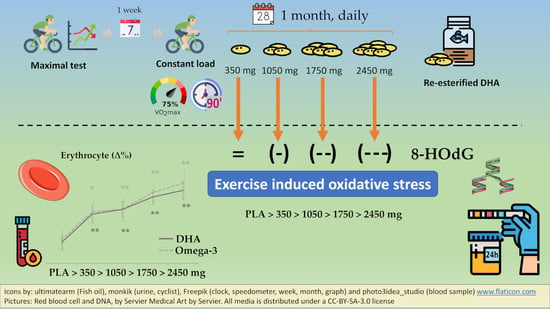
Oxidative Stress in Endurance Cycling Is Reduced Dose-Dependently after One Month of Re-Esterified DHA Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Subjects
2.3. Supplementation Protocol and Dietary Assessment
2.4. Exercise Test
2.4.1. Initial Physical Assessment: Aerobic Capacity and Health Assessment
2.4.2. Exercise-Induced Oxidative Stress Cycling Protocol
2.5. Biochemical Analysis and Markers
2.5.1. Endogenous Antioxidant Effect of rDHA
2.5.2. DHA and PUFA Incorporation to Erythrocyte
2.6. Statistical Analysis
3. Results
3.1. Participant Flow Diagram and Baseline Characteristics
3.2. Physical Tests
3.3. Nutritional Assessment
3.4. Endogenous Antioxidant Effect of rDHA
3.5. DHA and PUFA Incorporation to Erythrocyte
4. Discussion
4.1. Main Finding: rDHA Reduces Constant-Load Medium-Intensity Aerobic Exercise-Induced Oxidative Stress Dose-Dependently
4.2. Secondary Finding: DHA Is Incorporated to Erythrocytes Dose-Dependently
4.3. Contextualization of the Findings
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Fruits | Vegetables | Grain | Oils | Animal Products |
|---|---|---|---|---|
| Strawberries | Pepper | Coffee | Seed oils | Fish |
| Raspberries | Carrot | Green Tea | Fish oils | |
| Raspberries | Tomato | Cocoa | Seaweed oils | |
| Grapes | Lettuce | Cereals | ||
| Kiwis | Spinach | Corn | ||
| Blueberries | Onion | Nuts | ||
| Plums | Garlic | Walnuts | ||
| Orange | Soy and derivates | |||
| Lemon | Wheat germs | |||
| Papaya | ||||
| Apples | ||||
| Pineapple | ||||
| Avocado | ||||
| Red wine |
| Group | VO2 Max mL/min | VO2 Max mL/min kg | VT 2 mL/min | VT 2 mL/min kg |
|---|---|---|---|---|
| Placebo | 3390.9 ± 354.9 | 45.1 ± 5.6 | 2559.5 ± 296.7 | 34.2 ± 5.2 |
| 350 mg | 3607.7 ± 439.5 | 48.2 ± 5.2 | 2702.2 ± 326.9 | 36.2 ± 4.8 |
| 1050 mg | 3430.5 ± 274.5 | 45.0 ± 5.7 | 2666.5 ± 423.3 | 34.9 ± 5.7 |
| 1750 mg | 3590.0 ± 728.8 | 47.2 ± 9.7 | 2737.6 ± 452.0 | 35.9 ± 6.2 |
| 2450 mg | 3417.9 ± 602.5 | 43.1 ± 6.9 | 2873.9 ± 370.3 | 36.4 ± 5.1 |
| Total | 3487.4 ± 492.5 | 45.8 ± 6.8 | 2693.9 ± 377.2 | 35.4 ± 5.3 |
| Group | Trial | Water Consumption (mL) |
|---|---|---|
| Placebo | Day 0 | 1043.8 ± 756.5 |
| Day 28 | 948.1 ± 599.5 | |
| 350 mg | Day 0 | 590.0 ± 307.1 |
| Day 28 | 791.0 ± 535.4 | |
| 1050 mg | Day 0 | 601.8 ± 358.7 |
| Day 28 | 680.0 ± 417.4 | |
| 1750 mg | Day 0 | 967.1 ± 550.2 |
| Day 28 | 1214.2 ± 642.5 | |
| 2450 mg | Day 0 | 568.8 ± 243.4 |
| Day 28 | 400.0 ± 181.3 |
| Group | Trial | Time | Heart Rate (bpm) |
|---|---|---|---|
| Placebo | Day 0 | 30 min | 157.9 ± 11.5 |
| 60 min | 154.7 ± 12.5 | ||
| 90 min | 155.7 ± 10.4 | ||
| Day 28 | 30 min | 154.7 ± 12.1 | |
| 60 min | 154.9 ± 10.7 | ||
| 90 min | 155.1 ± 8.4 | ||
| 350 mg | Day 0 | 30 min | 152.8 ± 8.6 |
| 60 min | 152.8 ± 8.0 | ||
| 90 min | 149.9 ± 7.1 | ||
| Day 28 | 30 min | 151.3 ± 8.2 | |
| 60 min | 150.1 ± 9.1 | ||
| 90 min | 155.8 ± 14.9 | ||
| 1050 mg | Day 0 | 30 min | 158.1 ± 15.4 |
| 60 min | 158.1 ± 13.8 | ||
| 90 min | 157.8 ± 7.4 | ||
| Day 28 | 30 min | 157.4 ± 10.9 | |
| 60 min | 157.0 ± 11.0 | ||
| 90 min | 157.9 ± 11.5 | ||
| 1750 mg | Day 0 | 30 min | 154.7 ± 12.5 |
| 60 min | 155.7 ± 10.4 | ||
| 90 min | 154.7 ± 12.1 | ||
| Day 28 | 30 min | 154.9 ± 10.7 | |
| 60 min | 155.1 ± 8.4 | ||
| 90 min | 152.8 ± 8.6 | ||
| 2450 mg | Day 0 | 30 min | 149.9 ± 7.1 |
| 60 min | 151.3 ± 8.2 | ||
| 90 min | 150.1 ± 9.1 | ||
| Day 28 | 30 min | 155.8 ± 14.9 | |
| 60 min | 158.1 ± 15.4 | ||
| 90 min | 158.1 ± 13.8 |
References
- DeFilippis, A.P.; Sperling, L.S. Understanding omega-3′s. Am. Heart J. 2006, 151, 564–570. [Google Scholar] [CrossRef]
- Jeromson, S.; Gallagher, I.J.; Galloway, S.D.R.; Hamilton, D.L. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Domingo, P.; Fernández, I.; Gallego-Escuredo, J.M.; Torres, F.; Del Mar Gutierrez, M.; Mateo, M.G.; Villarroya, J.; Giralt, M.; Vidal, F.; Villarroya, F.; et al. Effects of docosahexanoic acid on metabolic and fat parameters in HIV-infected patients on cART: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2018, 37, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Gorjão, R.; Azevedo-Martins, A.K.; Rodrigues, H.G.; Abdulkader, F.; Arcisio-Miranda, M.; Procopio, J.; Curi, R. Comparative effects of DHA and EPA on cell function. Pharmacol. Ther. 2009, 122, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hilleman, D.; Smer, A. Prescription Omega-3 Fatty Acid Products and Dietary Supplements are not Interchangeable. Manag. Care 2016, 25, 46–52. [Google Scholar] [PubMed]
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Véricel, E.; Polette, A.; Bacot, S.; Calzada, C.; Lagarde, M. Pro- and antioxidant activities of docosahexaenoic acid on human blood platelets. J. Thromb. Haemost. 2003, 1, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Arab, K.; Rossary, A.; Flourié, F.; Tourneur, Y.; Steghens, J.-P. Docosahexaenoic acid enhances the antioxidant response of human fibroblasts by upregulating γ-glutamyl-cysteinyl ligase and glutathione reductase. Br. J. Nutr. 2006, 95, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesavulu, M.M.; Kameswararao, B.; Apparao, C.; Kumar, E.G.T.V.; Harinarayan, C.V. Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab. 2002, 28, 20–26. [Google Scholar]
- Ateya, A.M.; Sabri, N.A.; El Hakim, I.; Shaheen, S.M. Effect of Omega-3 Fatty Acids on Serum Lipid Profile and Oxidative Stress in Pediatric Patients on Regular Hemodialysis: A Randomized Placebo-Controlled Study. J. Ren. Nutr. 2017, 27, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, N.; Mekki, K.; Boukaddoum, A.; Dida, N.; Kaddous, A.; Bouchenak, M. Effects of Omega-3 Polyunsaturated Fatty-Acid Supplementation on Redox Status in Chronic Renal Failure Patients with Dyslipidemia. J. Ren. Nutr. 2010, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Tayyebi-Khosroshahi, H.; Houshyar, J.; Tabrizi, A.; Vatankhah, A.-M.; Zonouz, N.R.; Dehghan-Hesari, R. Effect of Omega-3 Fatty Acid on Oxidative Stress in Patients on Hemodialysis. Iran J. Kidney Dis. 2010, 4, 322–326. [Google Scholar] [PubMed]
- Gasso, F.; Bogdanov, P.; Domingo, J. Docosahexaenoic Acid Improves Endogen Antioxidant Defense in Arpe-19 Cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5932. [Google Scholar]
- Wu, W.H.; Lu, S.C.; Wang, T.F.; Jou, H.J.; Wang, T.A. Effects of docosahexaenoic acid supplementation on blood lipids, estrogen metabolism, and in vivo oxidative stress in postmenopausal vegetarian women. Eur. J. Clin. Nutr. 2006, 60, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Still, J.D.; Powers, C.A.; Hoffman, D.R.; Boyd-Trull, K.; Lester, L.A.; Benisek, D.C.; Arterburn, L.M. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: A randomized, controlled study. Nutrition 2006, 22, 36–46. [Google Scholar] [CrossRef]
- Shoji, H.; Franke, C.; Campoy, C.; Rivero, M.; Demmelmair, H.; Koletzko, B. Effect of docosahexaenoic acid and eicosapentaenoic acid supplementation on oxidative stress levels during pregnancy. Free Radic. Res. 2006, 40, 379–384. [Google Scholar] [CrossRef]
- Nitta, H.; Kinoyama, M.; Watanabe, A.; Shirao, K.; Kihara, H.; Arai, M. Effects of nutritional supplementation with antioxidant vitamins and minerals and fish oil on antioxidant status and psychosocial stress in smokers: An open trial. Clin. Exp. Med. 2007, 7, 179–183. [Google Scholar] [CrossRef]
- Mas, E.; Woodman, R.J.; Burke, V.; Puddey, I.B.; Beilin, L.J.; Durand, T.; Mori, T.A. The omega-3 fatty acids EPA and DHA decrease plasma F2-isoprostanes: Results from two placebo-controlled interventions. Free Radic. Res. 2010, 44, 983–990. [Google Scholar] [CrossRef]
- Mori, T.A.; Puddey, I.B.; Burke, V.; Croft, K.D.; Dunstan, D.W.; Rivera, J.H.; Beilin, L.J. Effect of ω3 fatty acids on oxidative stress in humans: GC–MS measurement of urinary F2-isoprostane excretion. Redox Rep. 2000, 5, 45–46. [Google Scholar] [CrossRef]
- Dyerberg, J.; Madsen, P.; Møller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef]
- Vollaard, N.B.J.; Shearman, J.P.; Cooper, C.E. Exercise-induced oxidative stress: Myths, realities and physiological relevance. Sports Med. 2005, 35, 1045–1062. [Google Scholar] [CrossRef] [PubMed]
- Alessio, H. Exercise-induced oxidative stress. Med. Sci. Sports Exerc. 1993, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Hrádková, I.; Merkl, R.; Šmidrkal, J.; Kyselka, J.; Filip, V. Antioxidant effect of mono- and dihydroxyphenols in sunflower oil with different levels of naturally present tocopherols. Eur. J. Lipid Sci. Technol. 2013, 115, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Tasan, M.; Demirci, M. Total and individual tocopherol contents of sunflower oil at different steps of refining. Eur. Food Res. Technol. 2005, 220, 251–254. [Google Scholar] [CrossRef]
- Skinner, J.S.; Mclellan, T.H. The Transition from Aerobic to Anaerobic Metabolism. Res. Q. Exerc. Sport 1980, 51, 234–248. [Google Scholar] [CrossRef]
- Arbor Assays, Inc. DNA Damage ELISA Kit. Available online: https://www.arborassays.com/product/dna-damage-eia-kit/ (accessed on 22 October 2020).
- Park, E.M.; Shigenaga, M.K.; Degan, P.; Korn, T.S.; Kitzler, J.W.; Wehr, C.M.; Kolachana, P.; Ames, B.N. Assay of excised oxidative DNA lesions: Isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc. Natl. Acad. Sci. USA 1992, 89, 3375–3379. [Google Scholar] [CrossRef] [Green Version]
- Osawa, T.; Yoshida, A.; Kawakishi, S.; Yamashita, K.; Ochi, H. Protective role of dietary antioxidants in oxidative stress. In Oxidative Stress and Aging. Molecular and Cell Biology Updates; Cutler, R.G., Packer, L., Bertram, J., Mori, A., Eds.; Birkhäuser: Basel, Switzerland, 1995; pp. 367–377. [Google Scholar]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid. Res. 1986, 27, 114–120. [Google Scholar]
- Zanón-Moreno, V.; Pedrol, J.D.; Sanz-González, S.; Raga-Cervera, J.; Salazar-Corral, J.; Pinazo-Durán, M.D. Feasibility study of a docosahexaenoic acid optimized nutraceutical formulation on the macular levels of lutein in a healthy Mediterranean population. Ophthalmic Res. 2020. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Birch, E.E.; Birch, D.G.; Uauy, R.; Castañeda, Y.S.; Lapus, M.G.; Wheaton, D.H. Impact of Early Dietary Intake and Blood Lipid Composition of Long-Chain Polyunsaturated Fatty Acids on Later Visual Development. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sato, M.; Kurotani, K.; Nanri, A.; Kawai, K.; Kasai, H.; Imaizumi, K.; Mizoue, T. PUFAs in serum cholesterol ester and oxidative DNA damage in Japanese men and women. Am. J. Clin. Nutr. 2012, 95, 1209–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-Isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10, 10–23. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, S.R.; Nieman, D.C.; Fox-Rabinovich, M.; Duran, V.; Mcanulty, L.S.; Henson, D.A.; Jin, F.; Landram, M.J. Effect of n-3 Fatty Acids and Antioxidants on Oxidative Stress after Exercise. Med. Sci. Sports Exerc. 2010, 42, 1704–1711. [Google Scholar] [CrossRef] [Green Version]
- Mori Trevor, A.; Bao Danny, Q.; Burke, V.; Puddey, I.B.; Beilin, L.J. Docosahexaenoic Acid but not Eicosapentaenoic Acid Lowers Ambulatory Blood Pressure and Heart Rate in Humans. Hypertension 1999, 34, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Dunstan, D.W.; Mori, T.A.; Puddey, I.B.; Beilin, L.J.; Burke, V.; Morton, A.R.; Stanton, K.G. The Independent and Combined Effects of Aerobic Exercise and Dietary Fish Intake on Serum Lipids and Glycemic Control in NIDDM: A randomized controlled study. Diabetes Care 1997, 20, 913–921. [Google Scholar] [CrossRef]
- Morrow, J.D.; Roberts, L.J. The isoprostanes: Unique bioactive products of lipid peroxidation. Prog. Lipid Res. 1997, 36, 1–21. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.; Stillwell, W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem. Phys. Lipids 2008, 153, 47–56. [Google Scholar] [CrossRef]
- Watkins, S.M.; Carter, L.C.; German, J.B. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J. Lipid Res. 1998, 39, 1583–1588. [Google Scholar]
- Powers, S.K.; DeRuisseau, K.C.; Quindry, J.; Hamilton, K.L. Dietary antioxidants and exercise. J. Sports Sci. 2004, 22, 81–94. [Google Scholar] [CrossRef]
- Abdukeyum, G.G.; Owen, A.J.; Larkin, T.A.; McLennan, P.L. Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat. J. Clin. Med. 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Lopes, P.A.; Martins, S.V.; Ferreira, J.; Alfaia, C.M.; Rolo, E.A.; Correia, J.J.; Pinto, R.M.A.; Ramos-Bueno, R.P.; Batista, I.; et al. Docosahexaenoic acid at the sn-2 position of structured triacylglycerols improved n-3 polyunsaturated fatty acid assimilation in tissues of hamsters. Nutr. Res. 2016, 36, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Neubronner, J.; Schuchardt, J.P.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur. J. Clin. Nutr. 2011, 65, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Neubronner, J.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Moderate doses of EPA and DHA from re-esterified triacylglycerols but not from ethyl-esters lower fasting serum triacylglycerols in statin-treated dyslipidemic subjects: Results from a six month randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D.R. Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 2018, 27, 1–19. [Google Scholar] [CrossRef]
- Harris, W.S.; Sands, S.A.; Windsor, S.L.; Ali Hakim, A.; Stevens, T.L.; Magalski, A.; Porter, C.B.; Borkon, A.M. Omega-3 Fatty Acids in Cardiac Biopsies From Heart Transplantation Patients. Circulation 2004, 110, 1645–1649. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Peternelj, T.-T.; Coombes, J.S. Antioxidant Supplementation during Exercise Training: Beneficial or detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Cabrera, M.C.; Ristow, M.; Viña, J. Antioxidant supplements in exercise: Worse than useless? Am. J. Physiol. Endocrinol. Metab. 2012, 302, E476–E477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, e2082145. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.C.; van Doornen, L.J.P.; Kemper, H.C.G. The Role of Antioxidant Vitamins and Enzymes in the Prevention of Exercise-Induced Muscle Damage. Sports Med. 1996, 21, 213–238. [Google Scholar] [CrossRef]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in Exercise Performance and Prevention of Exercise-Induced Muscle Damage. Oxidative Med. Cell Longev. 2013, 2013, 825928. [Google Scholar] [CrossRef] [Green Version]
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and Performance: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1589–1599. [Google Scholar] [CrossRef]



| Group | Age (Years) | Training Time (h) | Cycling (h) | VO2 Max (mL/min kg) | Weight (kg) |
|---|---|---|---|---|---|
| Placebo | 38.6 ± 12.2 | 8.3 ± 7.3 | 6.3 ± 3.8 | 45.1 ± 5.6 | 75.8 ± 9.3 |
| 350 mg | 36.9 ± 5.6 | 9.7 ± 2.0 | 8.5 ± 2.5 | 48.2 ± 5.2 | 74.9 ± 5.7 |
| 1050 mg | 37.8 ± 11.2 | 9.1 ± 2.7 | 6.9 ± 2.2 | 45.0 ± 5.7 | 76.7 ± 5.9 |
| 1750 mg | 40.9 ± 8.9 | 9.2 ± 5.1 | 8.5 ± 5.3 | 47.2 ± 9.7 | 76.3 ± 5.0 |
| 2450 mg | 38.4 ± 10.4 | 9.6 ± 3.5 | 8.0 ± 3.9 | 43.1 ± 6.9 | 79.9 ± 12.7 |
| ANOVA p-value | 0.909 | 0.961 | 0.518 | 0.536 | 0.867 |
| Group | Energy (Kcal) | Vitamin C (mg) | Vitamin E (mg) | Zinc (mg) | PUFA (g) | Omega-6 (g) | Omega-3 (g) |
|---|---|---|---|---|---|---|---|
| Placebo | 2512 ± 685 | 513 ± 490 | 8.1 ± 11.4 | 8.1 ± 10.9 | 15.5 ± 9.3 | 4.1 ± 2.0 | 1.1 ± 1.1 |
| 350 mg | 2302 ± 627 | 396 ± 550 | 6.4 ± 6.4 | 10.5 ± 7.9 | 18.6 ± 18.7 | 5.8 ± 3.5 | 1.0 ± 1.1 |
| 1050 mg | 2646 ± 837 | 336 ± 204 | 5.0 ± 5.5 | 8.5 ± 6.3 | 14.2 ± 9.1 | 4.5 ± 3.4 | 1.4 ± 1.4 |
| 1750 mg | 2731 ± 916 | 463 ± 464 | 7.2 ± 11.5 | 10.8 ± 8.8 | 15.9 ± 7.0 | 3.6 ± 1.9 | 1.8 ± 1.3 |
| 2450 mg | 2484 ± 356 | 392 ± 395 | 7.5 ± 5.5 | 9.5 ± 7.5 | 11.8 ± 3.8 | 3.1 ± 0.8 | 0.9 ± 0.8 |
| ANOVA p-value | 0.745 | 0.905 | 0.794 | 0.927 | 0.770 | 0.256 | 0.651 |
| Group | Time | Basal (ng/kg at 24 h) | Oxidative Stress (ng/kg at 24 h) | rDHA Antioxidant Effect (F-Snedecor) | ANOVA (Ox. Damage Time) |
|---|---|---|---|---|---|
| Placebo | Day 0 | 3965 ± 388 | 2067 ± 1894 ** | 80 ± 1400 (F = 0.041) | p = 0.026 |
| Day 28 | 4233 ± 453 | 1987 ± 1387 ** | |||
| 350 mg | Day 0 | 4084 ± 442 | 1391 ± 1768 ** | 234 ± 1526 (F = 0.268) | |
| Day 28 | 4388 ± 517 | 1157 ± 1428 ** | |||
| 1050 mg | Day 0 | 3328 ± 422 | 1546 ± 1360 ** | 1148 ± 1690 * (F = 7.112) | |
| Day 28 | 3418 ± 493 | 398 ± 959 | |||
| 1750 mg | Day 0 | 3924 ± 404 | 1757 ± 1621 ** | 1282 ± 1247 ** (F = 9.681) | |
| Day 28 | 4119 ± 472 | 475 ± 1083 | |||
| 2450 mg | Day 0 | 4352 ± 494 | 1734 ± 854 ** | 1970 ± 1179 ** (F = 15.230) | |
| Day 28 | 4472 ± 578 | −236 ± 765 |
| Group | Time | PUFA | Omega-3 | Omega-6 | n-6/n-3 Ratio | DHA | EPA |
|---|---|---|---|---|---|---|---|
| Placebo | Day 0 | 32.2 ± 1.4 | 5.6 ± 0.9 | 26.7 ± 1.1 | 4.9 ± 0.8 | 3.7 ± 0.6 | 0.46 ± 0.22 |
| Day 28 | 32.4 ± 2.0 | 5.6 ± 1.1 | 27.0 ± 1.3 | 5.0 ± 1.0 | 3.7 ± 0.7 | 0.45 ± 0.17 | |
| 350 mg | Day 0 | 32.3 ± 1.2 | 5.9 ± 1.6 | 26.4 ± 1.5 | 4.8 ± 1.4 | 3.9 ± 1.1 | 0.53 ± 0.23 |
| Day 28 | 32.3 ± 3.8 | 6.7 ± 1.6 * | 25.7 ± 3.3 | 4.0 ± 1.0 * | 4.6 ± 1.2 ** | 0.56 ± 0.21 | |
| 1050 mg | Day 0 | 32.1 ± 1.3 | 5.8 ± 1.8 | 26.4 ± 2.6 | 5.0 ± 1.7 | 3.7 ± 0.8 | 0.70 ± 0.71 |
| Day 28 | 32.0 ± 1.5 | 6.6 ± 1.9 ** | 25.4 ± 2.3 * | 4.2 ± 1.4 ** | 4.5 ± 0.9 ** | 0.81 ± 0.60 | |
| 1750 mg | Day 0 | 32.3 ± 1.2 | 5.4 ± 1.2 | 26.9 ± 1.3 | 5.4 ± 1.8 | 3.6 ± 0.8 | 0.44 ± 0.23 |
| Day 28 | 32.5 ± 1.4 | 6.6 ± 1.0 ** | 25.9 ± 1.4 * | 4.0 ± 0.9 ** | 4.7 ± 0.8 ** | 0.64 ± 0.20 | |
| 2450 mg | Day 0 | 32.9 ± 1.1 | 6.2 ± 1.5 | 26.7 ± 1.5 | 4.7 ± 2.1 | 4.2 ± 1.0 | 0.51 ± 0.26 |
| Day 28 | 33.3 ± 1.4 | 7.7 ± 1.2 ** | 25.7 ± 1.5 | 3.5 ± 0.9 ** | 5.4 ± 0.9 ** | 0.72 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Salazar, L.; Contreras, C.; Torregrosa-García, A.; Luque-Rubia, A.J.; Ávila-Gandía, V.; Domingo, J.C.; López-Román, F.J. Oxidative Stress in Endurance Cycling Is Reduced Dose-Dependently after One Month of Re-Esterified DHA Supplementation. Antioxidants 2020, 9, 1145. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111145
de Salazar L, Contreras C, Torregrosa-García A, Luque-Rubia AJ, Ávila-Gandía V, Domingo JC, López-Román FJ. Oxidative Stress in Endurance Cycling Is Reduced Dose-Dependently after One Month of Re-Esterified DHA Supplementation. Antioxidants. 2020; 9(11):1145. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111145
Chicago/Turabian Stylede Salazar, Lydia, Carlos Contreras, Antonio Torregrosa-García, Antonio J. Luque-Rubia, Vicente Ávila-Gandía, Joan Carles Domingo, and Francisco Javier López-Román. 2020. "Oxidative Stress in Endurance Cycling Is Reduced Dose-Dependently after One Month of Re-Esterified DHA Supplementation" Antioxidants 9, no. 11: 1145. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9111145