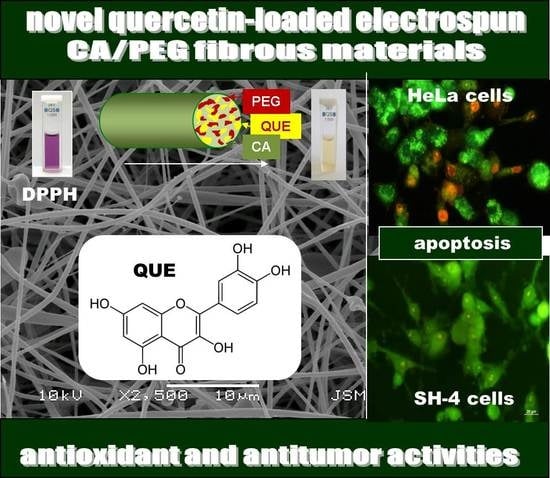
Antioxidant and Antitumor Activities of Novel Quercetin-Loaded Electrospun Cellulose Acetate/Polyethylene Glycol Fibrous Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fibrous Materials by Electrospinning
2.3. Characterization of the Fibrous Materials
2.4. MTT Cytotoxicity Assay
2.5. Study of the Effect of the Fibrous Mats on HeLa and SH-4 Cells Using Fluorescence Microscopy
Double Staining Assay with AO–EtBr
2.6. DAPI-Staining
3. Results
3.1. Preparation of Fibrous Mats by Electrospinning
3.2. IR Spectra of Fibrous Materials
3.3. Water Contact Angle
3.4. X-ray Diffraction Analysis
3.5. Thermal Characteristics of the Fibrous Mats
3.6. In Vitro Release Profile of Quercetin
3.7. Evaluation of the Antioxidant Activity
3.8. MTT Cytotoxicity Assay
3.9. Double Staining Assay with AO–EtBr
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- David, A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Lakhanpal, P.; Rai, D.K. Quercetin: A Versatile Flavonoid. Internet J. Med. Updat. 2007, 2, 22–37. [Google Scholar] [CrossRef] [Green Version]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, S.; Kilinc, O.; Selamoglu, Z. Antioxidant Activity of Quercetin: A Mechanistic Review. Turk. J. Agric. Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef] [Green Version]
- Mihaylova, D.; Schalow, S. Antioxidant and stabilization activity of a quercetin-containing flavonoid extract obtained from Bulgarian Sophora japonica L. Braz. Arch. Biol. Technol. 2013, 56, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Jaisinghani, R. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of anti-Staphylococcus aureus activity of quercetin. Int. J. Food Sci. Tech. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, L.; Liao, J.; Li, L.; Yao, W.; Xiong, Z.; Zhou, X. Investigation of the anti-cancer effect of quercetin on HepG2 cells in vivo. PLoS ONE 2017, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Review: Quercetin and its anti-allergic immune response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal nanocarriers for enhanced skin delivery of quercetin with functions of anti-tyrosinase and antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, V.S.S.; Rodríguez-Rojo, S.; De Paz, E.; Mato, C.; Martín, A.; Cocero, M.J. Production of water soluble quercetin formulations by pressurized ethyl acetate-in-water emulsion technique using natural origin surfactants. Food Hydrocoll. 2015, 51, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Madaan, K.; Lather, V.; Pandita, D. Evaluation of polyamidoamine dendrimers as potential carriers for quercetin, a versatile flavonoid. Drug Deliv. 2016, 23, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.; Chu, L.; Xia, Q. Liposome-chitosan hydrogel bead delivery system for the encapsulation of linseed oil and quercetin: Preparation and in vitro characterization studies. LWT Food Sci. Technol. 2020, 117, 108615. [Google Scholar] [CrossRef]
- Jangde, R.; Srivastava, S.; Singh, M.R.; Singh, D. In vitro and In vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int. J. Biol. Macromol. 2018, 115, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, E.J.; Bravo, J.M.C.; Medina, A.S.; Lizeth, G.; González, P.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Mat. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef]
- Toncheva, A.; Spasova, M.; Paneva, D.; Manolova, N.; Rashkov, I. Polylactide (PLA)-Based Electrospun Fibrous Materials Containing Ionic Drugs as Wound Dressing Materials: A Review. Int. J. Polym. Mater. Polym. Biomat. 2014, 63, 657–671. [Google Scholar] [CrossRef]
- Wang, G.; Su, Y.; Yu, J.; Li, R.; Ma, S.; Niu, X.; Shi, G. Preparation of Electrospun Active Molecular Membrane and Atmospheric Free Radicals Capture. Molecules 2019, 24, 3037. [Google Scholar] [CrossRef] [Green Version]
- Aytac, Z.; Ipek, S.; Durgun, E.; Uyar, T. Antioxidant electrospun zein nanofibrous web encapsulating quercetin/cyclodextrin inclusion complex. J. Mater. Sci. 2018, 53, 527–1539. [Google Scholar] [CrossRef] [Green Version]
- Eskitoros-Togay, Ş.M.; Emre Bulbul, Y.; Dilsiza, N. Quercetin-loaded and unloaded electrospun membranes: Synthesis, characterization and in vitro release study. J. Drug Deliv. Sci. Technol. 2018, 47, 22–30. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Yu, D.; Williams, G.R. Tunable biphasic drug release from ethyl cellulose nanofibers fabricated using a modified coaxial electrospinning process. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasband, W.S. (1997–2018) ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA. Available online: https://imagej.nih.gov/ij/ (accessed on 3 January 2016).
- Spasova, M.; Mincheva, R.; Paneva, D.; Manolova, N.; Rashkov, I. Perspectives on: Criteria for complex evaluation of the morphology and alignment of electrospun polymer nanofibers. J. Bioact. Compat. Polym. 2006, 21, 465–479. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tsekova, P.; Spasova, M.; Manolova, N.; Rashkov, I.; Markova, N.; Georgieva, A.; Toshkova, R. Еlectrospun cellulose acetate membranes decorated with curcumin-PVP particles: Preparation, antibacterial and antitumor activities. J. Mater. Sci. Mater. Med. 2018, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Sun, Y.; Qi, X.; Tan, F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech. 2012, 13, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; He, J.; Cui, S.; Gao, W. Studies of Electrospun Cellulose Acetate Nanofibrous Membranes. Open Mater. Sci. J. 2011, 5, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Telangea, D.; Wavarea, K.; Patila, A.; Umekara, M.; Anandb, S.; Daveb, V. Drug-phospholipid complex-loaded matrix film formulation for enhanced transdermal delivery of quercetin. J. Excip. Food Chem. 2018, 9, 31–50. [Google Scholar]
- Barud, H.; J’unior, A.M.; Santos, D.; de Assunc, R.M.N.; Meireles, C.; Cerqueira, D.; Filho, G.; Ribeiro, C.; Messaddeq, Y.; Ribeiro, S. Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Tungprapa, S.; Jangchud, I.; Supaphol, P. Release characteristics of four model drugs from drug-loaded electrospun cellulose acetate fiber mats. Polymer 2007, 48, 5030–5041. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Pol. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J.; Ghoul, M. Solubility of Flavonoids in Organic Solvents. J. Chem. Eng. Data 2007, 52, 1552–1556. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.; Howard, L.; Monrad, J. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Búzová, D.; Kasák, P.; Miškovský, P.; Jancura, D. Solubilization of poorly soluble photosensitizer hypericin by polymeric micelles and polyethylene glycol. Gen. Physiol. Biophys. 2013, 32, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.; Wang, Y.; Cui, Y. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Food 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Galluzzo, P.; Martini, C.; Bulzomi, P.; Leone, S.; Bolli, A.; Pallottini, V.; Marino, M. Quercetin-induced apoptotic cascade in cancer cells: Antioxidant versus estrogen receptor a-dependent mechanisms. Mol. Nutr. Food Res. 2009, 53, 699–708. [Google Scholar] [CrossRef]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Georgieva, A.; Toshkova, R. Antioxidant and Antitumor Activities of Novel Quercetin-Loaded Electrospun Cellulose Acetate/Polyethylene Glycol Fibrous Materials. Antioxidants 2020, 9, 232. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9030232
Stoyanova N, Spasova M, Manolova N, Rashkov I, Georgieva A, Toshkova R. Antioxidant and Antitumor Activities of Novel Quercetin-Loaded Electrospun Cellulose Acetate/Polyethylene Glycol Fibrous Materials. Antioxidants. 2020; 9(3):232. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9030232
Chicago/Turabian StyleStoyanova, Nikoleta, Mariya Spasova, Nevena Manolova, Iliya Rashkov, Ani Georgieva, and Reneta Toshkova. 2020. "Antioxidant and Antitumor Activities of Novel Quercetin-Loaded Electrospun Cellulose Acetate/Polyethylene Glycol Fibrous Materials" Antioxidants 9, no. 3: 232. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9030232