Antioxidant Properties of Casein Phosphopeptides (CPP) and Maillard-Type Conjugated Products
Abstract
:1. Introduction
2. Materials and Methods
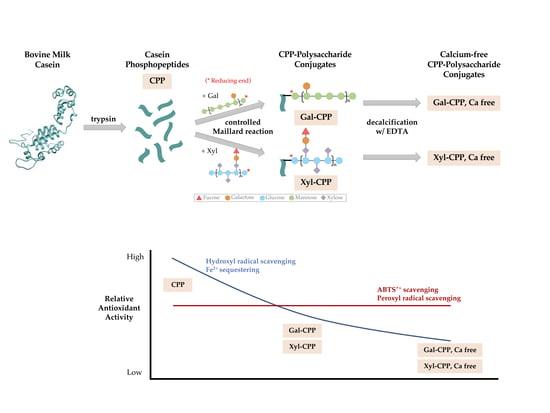
2.1. CPP, Polysaccharides, and CPP–Polysaccharide Conjugates
2.2. Molecular Weight
2.3. Determination of Antioxidant Capacity of CPP Preparation
2.4. Hydroxyl Radical Scavenging Assay in a Deoxyribose Model
2.5. Free Radical Scavenging Assay
2.6. Peroxyl Radical Scavenging Assays in a Liposome Model
2.7. Statistical Analysis
3. Results
3.1. Recovery of CPP
3.2. ORAC Analysis
3.3. Site-Specific and Non-Site-Specific Scavenging Activities
3.4. Scavenging Activity of ABTS Radicals by CPP and Conjugate Reactants
3.5. Effect of CPP and Conjugate Reactants on the Liposome Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H. Functional properties and applications of edible films made of milk proteins. J. Dairy Sci. 1995, 78, 2563–2583. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Conway, V.; Gauthier, S.F.; Pouliot, Y. Antioxidant activities of buttermilk proteins, whey proteins, and their enzymatic hydrolysates. J. Agric. Food Chem. 2013, 61, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrochano, A.R.; Sariçay, Y.; Arranz, E.; Kelly, P.M.; Buckin, V.; Giblin, L. Comparison of antioxidant activities of bovine whey proteins before and after simulated gastrointestinal digestion. J. Dairy Sci. 2019, 102, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-E.; Kim, J.W.; Cheon, S.; Nam, M.S.; Kim, K.K. Alpha-casein and beta-lactoglobulin from cow milk exhibit antioxidant activity: A plausible link to antiaging effects. J. Food Sci. 2019, 84, 3083–3090. [Google Scholar] [CrossRef]
- Díaz, M.; Dunn, C.M.; McClements, D.J.; Decker, E.A. Use of caseinophosphopeptides as natural antioxidants in oil-in-water emulsions. J. Agric. Food Chem. 2003, 51, 2365–2370. [Google Scholar] [CrossRef]
- Kitts, D.D. Antioxidant properties of casein-phosphopeptides. Trends Food Sci. Technol. 2005, 16, 549–554. [Google Scholar] [CrossRef]
- Petrat-Melin, B.; Andersen, P.; Rasmussen, J.T.; Poulsen, N.A.; Larsen, L.B.; Young, J.F. In vitro digestion of purified β-casein variants A1, A2, B, and I: Effects on antioxidant and angiotensin-converting enzyme inhibitory capacity. J. Dairy Sci. 2015, 98, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Rossini, K.; Noreña, C.P.Z.; Cladera-Olivera, F.; Brandelli, A. Casein peptides with inhibitory activity on lipid oxidation in beef homogenates and mechanically deboned poultry meat. LWT-Food Sci. Technol. 2009, 42, 862–867. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Ishihara, N.; Juneja, L.R. Antioxidant properties of casein calcium peptides and their effects on lipid oxidation in beef homogenates. J. Agric. Food Chem. 2005, 53, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Prakash, V. Bioactive peptides from bovine milk α-casein: Isolation, characterization and multifunctional properties. Int. J. Pept. Res. Ther. 2010, 16, 7–15. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Huang, G. Protein hydrolysates as promoters of non-haem iron absorption. Nutrients 2017, 9, 609. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Liao, W.; Pan, Z.; Wang, Q.; Duan, S.; Xiao, S.; Yang, Z.; Cao, Y. Isolation and identification of iron-chelating peptides from casein hydrolysates. Food Funct. 2019, 10, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.L.; Liu, Y.; Mels, J.; Dittrich, D.; Haus, S.; Gensberger-Reigl, S.; Pischetsrieder, M. Profiling of multiphosphorylated peptides in kefir and their release during simulated gastrointestinal digestion. ACS Omega 2019, 4, 7963–7970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smialowska, A.; Matia-Merino, L.; Carr, A.J. Oxidative stability of iron fortified goat and cow milk and their peptide isolates. Food Chem. 2017, 237, 1021–1024. [Google Scholar] [CrossRef]
- Bouhallab, S.; Bouglé, D. Biopeptides of milk: Caseinophosphopeptides and mineral bioavailability. Reprod. Nutr. Dev. 2004, 44, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.; Decker, E.A. Antioxidant mechanisms of caseinophosphopeptides and casein hydrolysates and their application in ground beef. J. Agric. Food Chem. 2004, 52, 8208–8213. [Google Scholar] [CrossRef]
- García-Nebot, M.J.; Barberá, R.; Alegría, A. Iron and zinc bioavailability in Caco-2 cells: Influence of caseinophosphopeptides. Food Chem. 2013, 138, 1298–1303. [Google Scholar] [CrossRef]
- Kansci, G.; Genot, C.; Meynier, A.; Gaucheron, F.; Chobert, J.-M. β-Caseinophosphopeptide (f1-25) confers on β-casein tryptic hydrolysate an antioxidant activity during iron/ascorbate-induced oxidation of liposomes. Le Lait 2004, 84, 449–462. [Google Scholar] [CrossRef]
- de Oliveira, F.C.; Coimbra, J.S.D.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Liu, Y.; Lin, D.; Qin, W.; Zhang, Z.; et al. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Nakamura, S. Production and use of Maillard products as oxidative stress modulators. J. Med. Food 2005, 8, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ogawa, M.; Nakai, S.; Kato, A.; Kitts, D.D. Antioxidant activity of a Maillard-type phosvitin–galactomannan conjugate with emulsifying properties and heat stability. J. Agric. Food Chem. 1998, 46, 3958–3963. [Google Scholar] [CrossRef]
- Gu, F.; Kim, J.M.; Hayat, K.; Xia, S.; Feng, B.; Zhang, X. Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a casein–glucose model system. Food Chem. 2009, 117, 48–54. [Google Scholar] [CrossRef]
- Tu, Y.; Xu, Y.; Ren, F.; Zhang, H. Characteristics and antioxidant activity of Maillard reaction products from α-lactalbumin and 2′-fucosyllactose. Food Chem. 2020, 316, 126341. [Google Scholar] [CrossRef]
- Xu, C.; Yu, S.; Yang, X.; Qi, J.; Lin, H.; Zhao, Z. Emulsifying properties and structural characteristics of β-conglycinin and dextran conjugates synthesised in a pressurised liquid system. Int. J. Food Sci. Technol. 2010, 45, 995–1001. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Physicochemical and emulsifying properties of whey protein isolate (WPI)−dextran conjugates produced in aqueous solution. J. Agric. Food Chem. 2010, 58, 2988–2994. [Google Scholar] [CrossRef]
- Sun, W.-W.; Yu, S.-J.; Yang, X.-Q.; Wang, J.-M.; Zhang, J.-B.; Zhang, Y.; Zheng, E.-L. Study on the rheological properties of heat-induced whey protein isolate–dextran conjugate gel. Food Res. Int. 2011, 44, 3259–3263. [Google Scholar] [CrossRef]
- Jin, B.; Zhou, X.; Li, B.; Chen, C.; Zhang, X.; Chen, S. Structure and antioxidant activity of soy protein isolate-dextran conjugates obtained by TiO2 photocatalysis. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Kato, A.; Kobayashi, K. Enhanced antioxidative effect of ovalbumin due to covalent binding of polysaccharides. J. Agric. Food Chem. 1992, 40, 2033–2037. [Google Scholar] [CrossRef]
- Nakamura, S.; Kato, A. Multi-functional biopolymer prepared by covalent attachment of galactomannan to egg-white proteins through naturally occurring Maillard reaction. Food/Nahrung 2000, 44, 201–206. [Google Scholar] [CrossRef]
- Hamdani, A.M.; Wani, I.A.; Bhat, N.A.; Siddiqi, R.A. Effect of guar gum conjugation on functional, antioxidant and antimicrobial activity of egg white lysozyme. Food Chem. 2018, 240, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.S.; Babiker, E.F.E.; Azakami, H.; Kato, A. Molecular mechanism of the excellent emulsifying properties of phosvitin−galactomannan conjugate. J. Agric. Food Chem. 1999, 47, 2262–2266. [Google Scholar] [CrossRef]
- Yang, J.-E.; Chun, S.-H.; Kim, H.H.; Choi, H.-D.; Lee, K.-W. Characterization of Maillard-type lysozyme-galactomannan conjugate having immune-enhancing effects. Food Chem. 2017, 227, 149–157. [Google Scholar] [CrossRef]
- Dickinson, E.; Hunt, J.A.; Dalgleish, D.G. Competitive adsorption of phosvitin with milk proteins in oil-in-water emulsions. Food Hydrocoll. 1991, 4, 403–414. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]
- Kitts, D.D.; Yuan, Y.V.; Nagasawa, T.; Moriyama, Y. Effect of casein, casein phosphopeptides and calcium intake on ileal 45Ca disappearance and temporal systolic blood pressure in spontaneously hypertensive rats. Br. J. Nutr. 1992, 68, 765–781. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC−fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Kumar, R. Importance of deoxyribose degradation assay for evaluating hydroxyl radical scavenging activity of Punica extract. Int. J. Food Prop. 2012, 15, 942–948. [Google Scholar] [CrossRef]
- Liang, N.; Dupuis, J.H.; Yada, R.Y.; Kitts, D.D. Chlorogenic acid isomers directly interact with Keap 1-Nrf2 signaling in Caco-2 cells. Mol. Cell Biochem. 2019, 457, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Kitts, D.D. Evaluation of antioxidant activity of epigallocatechin gallate in biphasic model systems in vitro. Mol. Cell Biochem. 2001, 218, 147–155. [Google Scholar] [CrossRef]
- Lesse, A.J.; Campagnari, A.A.; Bittner, W.E.; Apicella, M.A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 1990, 126, 109–117. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Riley, P.F.; Adamson, N.J. A selective precipitation purification procedure for multiple phosphoseryl-containing peptides and methods for their identification. Anal. Biochem. 1994, 217, 277–284. [Google Scholar] [CrossRef]
- Ono, T.; Takagi, Y.; Kunishi, I. Casein phosphopeptides from casein micelles by successive digestion with pepsin and trypsin. Biosci. Biotechol. Bioch. 1998, 62, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Ferraretto, A.; Signorile, A.; Gravaghi, C.; Fiorilli, A.; Tettamanti, G. Casein phosphopeptides influence calcium uptake by cultured human intestinal HT-29 tumor cells. J. Nutr. 2001, 131, 1655–1661. [Google Scholar] [CrossRef]
- Kim, G.-N.; Jang, H.-D.; Kim, C.-I. Antioxidant capacity of caseinophosphopeptides prepared from sodium caseinate using Alcalase. Food Chem. 2007, 104, 1359–1365. [Google Scholar] [CrossRef]
- Matalanis, A.; Decker, E.A.; McClements, D.J. Inhibition of lipid oxidation by encapsulation of emulsion droplets within hydrogel microspheres. Food Chem. 2012, 132, 766–772. [Google Scholar] [CrossRef]
- Rival, S.G.; Boeriu, C.G.; Wichers, H.J. Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J. Agric. Food Chem. 2001, 49, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.; Zhang, W. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Venuste, M.; Zhang, X.; Shoemaker, C.F.; Karangwa, E.; Abbas, S.; Kamdem, P.E. Influence of enzymatic hydrolysis and enzyme type on the nutritional and antioxidant properties of pumpkin meal hydrolysates. Food Funct. 2013, 4, 811–820. [Google Scholar] [CrossRef]
- Yuji, H.; Weiss, J.; Villeneuve, P.; López Giraldo, L.J.; Figueroa-Espinoza, M.-C.; Decker, E.A. Ability of surface-active antioxidants to inhibit lipid oxidation in oil-in-water emulsion. J. Agric. Food Chem. 2007, 55, 11052–11056. [Google Scholar] [CrossRef]
- Dos Santos, V.R.F.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Relationship between galactomannan structure and physicochemical properties of films produced thereof. J. Food Sci. Technol. 2015, 52, 8292–8299. [Google Scholar] [CrossRef] [Green Version]
- Pardeshi, C.V.; Kulkarni, A.D.; Belgamwar, V.S.; Surana, S.J. 7—Xyloglucan for drug delivery applications. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 143–169. ISBN 978-0-08-102194-1. [Google Scholar]
- Olsen, J.V.; Ong, S.-E.; Mann, M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell. Proteom. 2004, 3, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Teodorowicz, M.; van Neerven, J.; Savelkoul, H. Food Processing: The influence of the Maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef]
- Cross, K.J.; Huq, N.L.; Palamara, J.E.; Perich, J.W.; Reynolds, E.C. Physicochemical characterization of casein phosphopeptide-amorphous calcium phosphate nanocomplexes. J. Biol. Chem. 2005, 280, 15362–15369. [Google Scholar] [CrossRef] [Green Version]
- Cross, K.J.; Huq, N.L.; Bicknell, W.; Reynolds, E.C. Cation-dependent structural features of β-casein-(1–25). Biochem. J. 2001, 356, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.G.; Fry, S.C. Characteristics of xyloglucan after attack by hydroxyl radicals. Carbohydr. Res. 2001, 332, 389–403. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lemus, E.; Silva, E.; Barrias, P.; Aspee, A.; Escobar, E.; Lorentzen, L.G.; Carroll, L.; Leinisch, F.; Davies, M.J.; López-Alarcón, C. Aggregation of α- and β- caseins induced by peroxyl radicals involves secondary reactions of carbonyl compounds as well as di-tyrosine and di-tryptophan formation. Free Radic. Biol. Med. 2018, 124, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.H.; Catalá, Á.; Vignoni, M. Soybean phosphatidylcholine liposomes as model membranes to study lipid peroxidation photoinduced by pterin. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosca, M.; Ceglie, A.; Ambrosone, L. Effect of membrane composition on lipid oxidation in liposomes. Chem. Phys. Lipids 2011, 164, 158–165. [Google Scholar] [CrossRef]
- Kasran, M.; Cui, S.W.; Goff, H.D. Emulsifying properties of soy whey protein isolate–fenugreek gum conjugates in oil-in-water emulsion model system. Food Hydrocoll. 2013, 30, 691–697. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein—Polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.-H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Kato, A.; Mifuru, R.; Matsudomi, N.; Kobayashi, K. Functional casein-polysaccharide conjugates prepared by controlled dry heating. Biosci. Biotechnol. Biochem. 1992, 56, 567–571. [Google Scholar] [CrossRef]
- Shu, Y.-W.; Sahara, S.; Nakamura, S.; Kato, A. Effects of the length of polysaccharide chains on the functional properties of the Maillard-type lysozyme-polysaccharide conjugate. J. Agric. Food Chem. 1996, 44, 2544–2548. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Nakamura, S.; Kitts, D.D. Antioxidant Properties of Casein Phosphopeptides (CPP) and Maillard-Type Conjugated Products. Antioxidants 2020, 9, 648. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080648
Zhang H, Nakamura S, Kitts DD. Antioxidant Properties of Casein Phosphopeptides (CPP) and Maillard-Type Conjugated Products. Antioxidants. 2020; 9(8):648. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080648
Chicago/Turabian StyleZhang, Huiying, Soichiro Nakamura, and David D. Kitts. 2020. "Antioxidant Properties of Casein Phosphopeptides (CPP) and Maillard-Type Conjugated Products" Antioxidants 9, no. 8: 648. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080648