Comparative Study of Protective Action of Exogenous 2-Cys Peroxiredoxins (Prx1 and Prx2) Under Renal Ischemia-Reperfusion Injury
Abstract
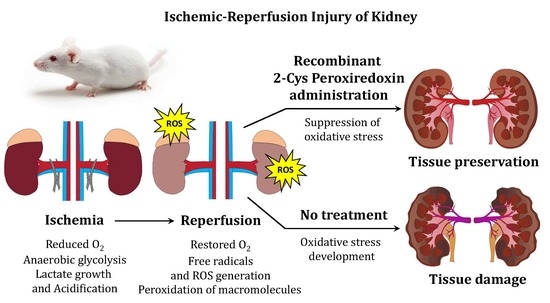
:1. Introduction
2. Materials and Methods
2.1. Gene Cloning and Enzyme Production
PRDX1m-R: 5′-CCTCCTCGAGCTTCTGCTTAGAGAAATACT-3′,
PRDX2m-F 5′-CGCACATATGGCCTCCGGCAACGCGCA-3′,
PRDX2m-R: 5′-CTACTCGAGGTTGTGTTTGGAGAAGTATTC-3′.
2.2. Determination of Peroxidase Activity of Enzymes
2.3. Determination of Thermal Stability of Enzymes
2.4. Determination of Endotoxins Level
2.5. Animals
2.6. Animal Model of Renal Ischemia-Reperfusion Injury
2.7. Histological Analysis
2.8. Electrophoresis and Immunoblotting
2.9. Gene Expression Level Analysis
2.10. Determination of MDA Level
2.11. Biochemical Blood Analysis
2.12. Statistical Data Analysis
3. Results
3.1. Estimation of the Circulation Time of Prx1 and Prx2 in the Bloodstream of Animals
3.2. Survival of Animals after I/R and with Prior Administration of Prx1 and Prx2
3.3. Histological Analysis of Kidney Tissue after I/R Injury and with Prior Administration of Prx1 and Prx2
3.4. Biochemical Analysis of Animal Blood after I/R Injury and Prior Administration of Prx1 and Prx2
3.5. MDA Level in the Renal Tissue after I/R and with Prior Administration of Prx1 and Prx2
3.6. Assessment of Gene Expression in the Kidney Tissue after I/R Injury and with Prior Administration of Prx1 and Prx2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrianova, N.V.; Buyan, M.I.; Zorova, L.D.; Pevzner, I.B.; Popkov, V.A.; Babenko, V.A.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Kidney cells regeneration: Dedifferentiation of tubular epithelium, resident stem cells and possible niches for renal progenitors. Int. J. Mol. Sci. 2019, 20, 6326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Ischemia–Reperfusion Injury of the Intestine and Protective Strategies Against Injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Inagaki, T.; Akiyama, T.; Du, C.-K.; Zhan, D.-Y.; Yoshimoto, M.; Shirai, M. Monoamine oxidase-induced hydroxyl radical production and cardiomyocyte injury during myocardial ischemia–reperfusion in rats. Free Radic. Res. 2016, 50, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.W.; Li, Y.; Kim, Y.M.; Sudhahar, V.; Abdelsaid, K.; Kim, H.W.; Liu, Y.; Fulton, D.J.R.; Ashraf, M.; Tang, Y.; et al. Modification of cardiac progenitor cell-derived exosomes by miR-322 provides protection against myocardial infarction through nox2-dependent angiogenesis. Antioxidants 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.E.; Lee, K.S.; Choi, K.H.; Kim, K.H.; Yang, S.C.; Han, W.K. Preconditioning strategies for kidney ischemia reperfusion injury: Implications of the “time-window” in remote ischemic preconditioning. PLoS ONE 2015, 10, e0124130. [Google Scholar] [CrossRef]
- Juhaszova, M.; Zorov, D.B.; Yaniv, Y.; Nuss, H.B.; Wang, S.; Sollott, S.J. Role of Glycogen Synthase Kinase-3 in Cardioprotection. Circ. Res. 2009, 104, 1240–1252. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, E.Y.; Zorov, D.B. Pros and cons of use of mitochondria-targeted antioxidants. Antioxidants 2019, 8, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksimenko, A.V.; Vavaev, A.V. Antioxidant enzymes as potential targets in cardioprotection and treatment of cardiovascular diseases. Enzyme antioxidants: The next stage of pharmacological counterwork to the oxidative stress. Hear. Int. 2012, 7, e3. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, A.E.; Temnov, A.A.; Charnagalov, A.A.; Sharapov, M.G.; Fesenko, E.E.; Novoselov, V.I. Protective Effect of Peroxiredoxin 6 in Ischemia/Reperfusion-Induced Damage of Small Intestine. Dig. Dis. Sci. 2015, 60, 3610–3619. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The role of peroxiredoxin 6 in neutralization of X-ray mediated oxidative stress: Effects on gene expression, preservation of radiosensitive tissues and postradiation survival of animals. Free Radic. Res. 2017, 51, 148–166. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Penkov, N.V.; Fesenko, E.E.; Vedunova, M.V.; Bruskov, V.I.; Gudkov, S.V. Protective and adaptogenic role of peroxiredoxin 2 (Prx2) in neutralization of oxidative stress induced by ionizing radiation. Free Radic. Biol. Med. 2019, 134, 76–86. [Google Scholar] [CrossRef]
- Goncharov, R.G.; Rogov, K.A.; Temnov, A.A.; Novoselov, V.I.; Sharapov, M.G. Protective role of exogenous recombinant peroxiredoxin 6 under ischemia-reperfusion injury of kidney. Cell Tissue Res. 2019, 378, 319–332. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Gudkov, S.V. Radioprotective Role of Peroxiredoxin 6. Antioxidants 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.G.; Kil, I.S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2016, 85, 1–27. [Google Scholar] [CrossRef]
- Arevalo, J.A.; Vázquez-Medina, J.P. The role of peroxiredoxin 6 in cell signaling. Antioxidants 2018, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Forshaw, T.E.; Holmila, R.; Nelson, K.J.; Lewis, J.E.; Kemp, M.L.; Tsang, A.W.; Poole, L.B.; Lowther, W.T.; Furdui, C.M. Peroxiredoxins in cancer and response to radiation therapies. Antioxidants 2019, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Radyuk, S.N.; Orr, W.C. The Multifaceted Impact of Peroxiredoxins on Aging and Disease. Antioxidants Redox Signal. 2018, 29, 1293–1311. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T. Close teamwork between Nrf2 and peroxiredoxins 1 and 6 for the regulation of prostaglandin D2 and E2 production in macrophages in acute inflammation. Free Radic. Biol. Med. 2015, 88, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.Z.; Kim, H.J.; Kang, S.W.; Rhee, S.G. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract. 1999, 45, 101–112. [Google Scholar] [CrossRef]
- Lee, W.; Choi, K.-S.; Riddell, J.; Ip, C.; Ghosh, D.; Park, J.-H.; Park, Y.-M. Human peroxiredoxin 1 and 2 are not duplicate proteins: The unique presence of Cys83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J. Biol. Chem. 2007, 282, 22011–22022. [Google Scholar] [CrossRef]
- Portillo-Ledesma, S.; Randall, L.M.; Parsonage, D.; Dalla Rizza, J.; Karplus, P.A.; Poole, L.B.; Denicola, A.; Ferrer-Sueta, G. Differential Kinetics of Two-Cysteine Peroxiredoxin Disulfide Formation Reveal a Novel Model for Peroxide Sensing. Biochemistry 2018, 57, 3416–3424. [Google Scholar] [CrossRef]
- Dalla Rizza, J.; Randall, L.M.; Santos, J.; Ferrer-Sueta, G.; Denicola, A. Differential parameters between cytosolic 2-Cys peroxiredoxins, PRDX1 and PRDX2. Protein Sci. 2019, 28, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, R.M.; Hughes, S.M.; Ledgerwood, E.C. Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic. Biol. Med. 2012, 53, 1522–1530. [Google Scholar] [CrossRef]
- Sobotta, M.C.; Liou, W.; Stöcker, S.; Talwar, D.; Oehler, M.; Ruppert, T.; Scharf, A.N.D.; Dick, T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015, 11, 64–70. [Google Scholar] [CrossRef]
- West, J.D.; Roston, T.J.; David, J.B.; Allan, K.M.; Loberg, M.A. Piecing together how peroxiredoxins maintain genomic stability. Antioxidants 2018, 7, 177. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Jang, H.H. Role of Cytosolic 2-Cys Prx1 and Prx2 in redox signaling. Antioxidants 2019, 8, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharapov, M.G.; Novoselov, V.I. Catalytic and Signaling Role of Peroxiredoxins in Carcinogenesis. Biochemistry 2019, 84, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.A.; Krause, D.S.; Carman, C.V.; Das, S.; Dubey, D.P.; Abraham, J.L.; Bronson, R.T.; Fujiwara, Y.; Orkin, S.H.; Van Etten, R.A. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 2003, 424, 561–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisucka, J.; Chauhan, A.K.; Patten, I.S.; Yesilaltay, A.; Neumann, C.; Van Etten, R.A.; Krieger, M.; Wagner, D.D. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circ. Res. 2008, 103, 598–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.-H.; Kim, S.H.; Yu, S.-L.; Kim, S.H.; Park, D.S.; Moon, H.-B.; Dho, S.H.; Kwon, K.-S.; Kwon, H.J.; Han, Y.-H.; et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 2003, 101, 5033–5038. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-H.; Kim, S.-U.; Kwon, T.-H.; Kim, J.-M.; Song, I.-S.; Shin, H.-J.; Lee, B.-K.; Bang, D.-H.; Lee, S.-J.; Lee, D.-S.; et al. Peroxiredoxin II promotes hepatic tumorigenesis through cooperation with Ras/Forkhead box M1 signaling pathway. Oncogene 2016, 35, 3503–3513. [Google Scholar] [CrossRef]
- Park, J.-G.; Yoo, J.-Y.; Jeong, S.-J.; Choi, J.-H.; Lee, M.-R.; Lee, M.-N.; Hwa Lee, J.; Kim, H.C.; Jo, H.; Yu, D.-Y.; et al. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circ. Res. 2011, 109, 739–749. [Google Scholar] [CrossRef]
- Schröder, E.; Brennan, J.P.; Eaton, P. Cardiac peroxiredoxins undergo complex modifications during cardiac oxidant stress. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H425–H433. [Google Scholar] [CrossRef] [Green Version]
- Bayer, S.B.; Low, F.M.; Hampton, M.B.; Winterbourn, C.C. Interactions between peroxiredoxin 2, hemichrome and the erythrocyte membrane. Free Radic. Res. 2016, 50, 1329–1339. [Google Scholar] [CrossRef]
- Matte, A.; Bertoldi, M.; Mohandas, N.; An, X.; Bugatti, A.; Brunati, A.M.; Rusnati, M.; Tibaldi, E.; Siciliano, A.; Turrini, F.; et al. Membrane association of peroxiredoxin-2 in red cells is mediated by the N-terminal cytoplasmic domain of band 3. Free Radic. Biol. Med. 2013, 55, 27–35. [Google Scholar] [CrossRef]
- Matte, A.; De Falco, L.; Federti, E.; Cozzi, A.; Iolascon, A.; Levi, S.; Mohandas, N.; Zamo, A.; Bruno, M.; Lebouef, C.; et al. Peroxiredoxin-2: A Novel Regulator of Iron Homeostasis in Ineffective Erythropoiesis. Antioxid. Redox Signal. 2018, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Hou, G.; Liu, H.; Zhang, M. Peroxiredoxin 1 promotes tumorigenesis through regulating the activity of mTOR/p70S6K pathway in esophageal squamous cell carcinoma. Med. Oncol. 2015, 32, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wei, J.; Zhang, S.; Wu, X.; Guo, J.; Liu, M.; Du, K.; Xu, J.; Peng, L.; Lv, Z.; et al. Peroxiredoxin 2 is essential for maintaining cancer stem cell-like phenotype through activation of Hedgehog signaling pathway in colon cancer. Oncotarget 2016, 7, 86816–86828. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.W.; Lee, S.; Lee, J.H.S. Cancer-associated function of 2-cys peroxiredoxin subtypes as a survival gatekeeper. Antioxidants 2018, 7, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharapov, M.G.; Novoselov, V.I.; Samygina, V.R.; Konarev, P.V.; Molochkov, A.V.; Sekirin, A.B.; Balkanov, A.S.; Gudkov, S.V. A chimeric recombinant protein with peroxidase and superoxide dismutase activities: Physico-chemical characterization and applicability to neutralize oxidative stress caused by ionizing radiation. Biochem. Eng. J. 2020, 159, 107603. [Google Scholar] [CrossRef]
- Kang, S.W.; Baines, I.C.; Rhee, S.G. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J. Biol. Chem. 1998, 273, 6303–6311. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Dong, Z. Mouse model of ischemic acute kidney injury: Technical notes and tricks. Am. J. Physiol. Ren. Physiol. 2012, 303, F1487–F1494. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar]
- Karaduleva, E.V.; Mubarakshina, E.K.; Sharapov, M.G.; Volkova, A.E.; Pimenov, O.Y.; Ravin, V.K.; Kokoz, Y.M.; Novoselov, V.I. Cardioprotective Effect of Modified Peroxiredoxins in Retrograde Perfusion of Isolated Rat Heart under Conditions of Oxidative Stress. Bull. Exp. Biol. Med. 2016, 160, 639–642. [Google Scholar] [CrossRef]
- Kosieradzki, M.; Rowiński, W. Ischemia/Reperfusion Injury in Kidney Transplantation: Mechanisms and Prevention. Transplant. Proc. 2008, 40, 3279–3288. [Google Scholar] [CrossRef]
- Baum, N.; Dichoso, C.C.; Carlton, C.E. Blood urea nitrogen and serum creatinine. Urology 1975, 5, 583–588. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney injury molecule-1: A translational Journey. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 293–299. [Google Scholar] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy, J.R.; Oesteritz, S.; Hanschmann, E.M.; Ockenga, W.; Ackermann, W.; Lillig, C.H. Segment-specific overexpression of redoxins after renal ischemia and reperfusion: Protective roles of glutaredoxin 2, peroxiredoxin 3, and peroxiredoxin 6. Free Radic. Biol. Med. 2011, 51, 552–561. [Google Scholar] [CrossRef]
- Aminzadeh, M.A.; Nicholas, S.B.; Norris, K.C.; Vaziri, N.D. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol. Dial. Transplant. 2013, 28, 2038–2045. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Ravin, V.K.; Novoselov, V.I. Peroxyredoxins as multifunctional enzymes. Mol. Biol. 2014, 48, 600–628. [Google Scholar] [CrossRef]
- Wu, C.L.; Su, T.C.; Chang, C.C.; Kor, C.T.; Chang, C.H.; Yang, T.H.; Chiu, P.F.; Tarng, D.C. Tubular Peroxiredoxin 3 as a Predictor of Renal Recovery from Acute Tubular Necrosis in Patients with Chronic Kidney Disease. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Halazun, K.J.; Al-Mukhtar, A.; Aldouri, A.; Willis, S.; Ahmad, N. Warm ischemia in transplantation: Search for a consensus definition. Transplant. Proc. 2007, 39, 1329–1331. [Google Scholar] [CrossRef]
- Hossain, M.A.; De Souza, A.I.; Bagul, A.; MacPhee, I.A.M.; Kessaris, N.; Morsy, M.A. HSP70, Peroxiredoxin-3 and -6 are upregulated during renal warm ischaemia in a donation after circulatory death model. J. Proteomics 2014, 108, 133–145. [Google Scholar] [CrossRef]
- Pires, B.R.B.; Silva, R.C.M.C.; Ferreira, G.M.; Abdelhay, E. NF-kappaB: Two sides of the same coin. Genes 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuther-Madrid, J.Y.; Kashatus, D.; Chen, S.; Li, X.; Westwick, J.; Davis, R.J.; Earp, H.S.; Wang, C.-Y.; Baldwin, A.S., Jr. The p65/RelA subunit of NF-kappaB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol. Cell. Biol. 2002, 22, 8175–8183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Tang, G.; Xiang, J.; Dai, Q.; Rosner, M.R.; Lin, A. The absence of NF-kappaB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 2002, 22, 8571–8579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biet, F.; Locht, C.; Kremer, L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J. Mol. Med. 2002, 80, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yin, M.J.; Lin, K.M.; Gaynor, R.B. Sulindac inhibits activation of the NF-kappaB pathway. J. Biol. Chem. 1999, 274, 27307–27314. [Google Scholar] [CrossRef] [Green Version]
- Palayoor, S.T.; Youmell, M.Y.; Calderwood, S.K.; Coleman, C.N.; Price, B.D. Constitutive activation of IκB kinase α and NF-κB in prostate cancer cells is inhibited by ibuprofen. Oncogene 1999, 18, 7389–7394. [Google Scholar] [CrossRef]
- Acarin, L.; González, B.; Castellano, B. Oral administration of the anti-inflammatory substance triflusal results in the downregulation of constitutive transcription factor NF-kappaB in the postnatal rat brain. Neurosci. Lett. 2000, 288, 41–44. [Google Scholar] [CrossRef]
- Staal, F.J.; Roederer, M.; Herzenberg, L.A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 1990, 87, 9943–9947. [Google Scholar] [CrossRef] [Green Version]
- Hanschmann, E.-M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins-molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxidants Redox Signal. 2013, 19, 1539–1605. [Google Scholar] [CrossRef]
- Vénéreau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xie, Y.; Sun, X.; Zeh Iii, H.J.; Kang, R.; Lotze, M.T.; Tang, D. DAMPs, Ageing, and Cancer: The “DAMP Hypothesis” HHS Public Access. Ageing Res. Rev. 2015, 24, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shichita, T.; Ago, T.; Kamouchi, M.; Kitazono, T.; Yoshimura, A.; Ooboshi, H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J. Neurochem. 2012, 123, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Wang, L.-F.; Yu, L.; Li, Y.-J.; Wang, Y.-N.; He, Q.; Chen, C.; Du, J.-R. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: Involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic. Biol. Med. 2014, 71, 165–175. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.-S.; Zhang, Z.-H.; Zhou, X.-M.; Gao, Y.-Y.; Liu, G.-J.; Wang, H.; Wu, L.-Y.; Li, W.; Hang, C.-H. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J. Neuroinflamm. 2018, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zheng, X.; Li, D.; Wang, T.; Xu, R.; Piao, H.; Liu, K. Prx1 promotes the proliferation and migration of vascular smooth muscle cells in a TLR4-dependent manner. Mol. Med. Rep. 2017, 15, 345–351. [Google Scholar] [CrossRef]
- Riddell, J.R.; Wang, X.-Y.; Minderman, H.; Gollnick, S.O. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J Immunol. 2010, 184, 1022–1030. [Google Scholar] [CrossRef]
- Knoops, B.; Becker, S.; Poncin, M.A.; Glibert, J.; Derclaye, S.; Clippe, A.; Alsteens, D. Specific Interactions Measured by AFM on Living Cells between Peroxiredoxin-5 and TLR4: Relevance for Mechanisms of Innate Immunity. Cell Chem. Biol. 2018, 1–10. [Google Scholar] [CrossRef]
- Jin, X.; Chen, C.; Li, D.; Su, Q.; Hang, Y.; Zhang, P.; Hu, W. PRDX2 in Myocyte Hypertrophy and Survival is Mediated by TLR4 in Acute Infarcted Myocardium. Sci. Rep. 2017, 7, 6970. [Google Scholar] [CrossRef]
- Park, K.M.; Byun, J.Y.; Kramers, C.; Kim, J.I.; Huang, P.L.; Bonventre, J.V. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J. Biol. Chem. 2003, 278, 27256–27266. [Google Scholar] [CrossRef] [Green Version]
- Ishimura, T.; Fujisawa, M.; Isotani, S.; Iijima, K.; Yoshikawa, N.; Kamidono, S. Endothelial nitric oxide synthase expression in ischemia-reperfusion injury after living related-donor renal transplantation. Transplant. Int. 2002, 15, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Li, J.J. The role of NF-kappaB in the regulation of cell stress responses. Int. Immunopharmacol. 2002, 2, 1509–1520. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Gordeeva, A.E.; Goncharov, R.G.; Tikhonova, I.V.; Ravin, V.K.; Temnov, A.A.; Fesenko, E.E.; Novoselov, V.I. The Effect of Exogenous Peroxiredoxin 6 on the State of Mesenteric Vessels and the Small Intestine in Ischemia–Reperfusion Injury. Biophysics 2017, 62, 998–1008. [Google Scholar] [CrossRef]
- Riddell, J.R.; Maier, P.; Sass, S.N.; Moser, M.T.; Foster, B.A.; Gollnick, S.O. Peroxiredoxin 1 stimulates endothelial cell expression of VEGF via TLR4 dependent activation of HIF-1α. PLoS ONE 2012, 7, e50394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valiente-Alandi, I.; Schafer, A.E.; Blaxall, B.C. Extracellular matrix-mediated cellular communication in the heart. J. Mol. Cell. Cardiol. 2016, 91, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Ihida-Stansbury, K.; McKean, D.M.; Gebb, S.A.; Martin, J.F.; Stevens, T.; Nemenoff, R.; Akeson, A.; Vaughn, J.; Jones, P.L. Paired-Related Homeobox Gene Prx1 Is Required for Pulmonary Vascular Development. Circ. Res. 2004, 94, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.P.; Chiquet-Ehrismann, R. Tenascin-C: Its functions as an integrin ligand. Int. J. Biochem. Cell Biol. 2015, 65, 165–168. [Google Scholar] [CrossRef]
- Ihida-Stansbury, K.; Ames, J.; Chokshi, M.; Aiad, N.; Sanyal, S.; Kawabata, K.C.; Levental, I.; Sundararaghavan, H.G.; Burdick, J.A.; Janmey, P.; et al. Role played by Prx1-dependent extracellular matrix properties in vascular smooth muscle development in embryonic lungs. Pulm. Circ. 2015, 5, 382–397. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.H.; Lee, I.K.; Kim, G.W.; Kim, B.U.; Han, Y.; Yu, D.; Park, H.S.; Kim, K.Y.; Lee, J.S.; Choi, C.; et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 2005, 435, 347–353. [Google Scholar] [CrossRef]
- Bae, Y.S.; Sung, J.Y.; Kim, O.S.; Kim, Y.J.; Hur, K.C.; Kazlauskas, A.; Rhee, S.G. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 2000, 275, 10527–10531. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, K.; Lei, Y.; Li, Q.; Nice, E.C.; Huang, C. Redox signaling: Potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic. Biol. Med. 2015, 89, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.A.J.; Sawicka, K.; Arcand, S.; O’Brien, P.; Luke, P.; Beck, G.; Sawicka, J.; Cohen, A.; Sawicki, G. Proteomic analysis of perfusate from machine cold perfusion of transplant kidneys: Insights into protection from injury. Ann. Transplant. 2017, 22, 730–739. [Google Scholar] [CrossRef] [PubMed]







| Genes | GenBank Accsession # | Oligonucleotides 5′-3′ (F + R) | Amplicon Size, bp |
|---|---|---|---|
| bAct | NM_007393.4 | CCTTCCTTCTTGGGTATGGAATCC CACCAGACAGCACTGTGTTGGCA | 115 |
| CASP3 | NM_009810 | AAGGAGCAGCTTTGTGTGTG GAAGAGTTTCGGCTTTCCAG | 145 |
| eNOS | NM_021838.2 | GAACCTGAGGGTGCCCAG TCCGATTCAACAGTGTCTCCT | 71 |
| iNOS | NM_012611.3 | GCTACACTTCCAACGCAACA CATGGTGAACACGTTCTTGG | 115 |
| IL-6 | NM_031168 | TAGTCCTTCCTACCCCAATTTCC TTGGTCCTTAGCCACTCCTTC | 76 |
| IL-18 | NM_008360.1 | GTGTTCCAGGACACAACAAG CTTCCTTTTGGCAAGCAAGA | 74 |
| NF-kB | NM_008689 | CCACGCTCAGCTTGTGAGGGAT GGCCAAGTGCAGAGGTGTCTGAT | 106 |
| NRF2 | NM_010902 | CTCGCTGGAAAAAGAAGTG CCGTCCAGGAGTTCAGAGG | 240 |
| KIM-1 | NM_001166632.1 | TTGCCTTCCGTGTCTCTAAG AGATGTTGTCTTCAGCTCGG | 225 |
| CAT | NM_009804 | AGCGACCAGATGAAGCAGTG TCCGCTCTCTGTCAAAGTGTG | 181 |
| PRDX1 | NM_011034 | AATGCAAAAATTGGGTATCCTGC CGTGGGACACACAAAAGTAAAGT | 149 |
| PRDX2 | NM_011563 | CACCTGGCGTGGATCAATACC GACCCCTGTAAGCAATGCCC | 138 |
| PRDX3 | NM_007452 | GGTTGCTCGTCATGCAAGTG CCACAGTATGTCTGTCAAACAGG | 99 |
| PRDX4 | NM_016764 | CTCAAACTGACTGACTATCGTGG CGATCCCCAAAAGCGATGATTTC | 101 |
| PRDX5 | NM_012021 | GGCTGTTCTAAGACCCACCTG GGAGCCGAACCTTGCCTTC | 154 |
| PRDX6 | NM_007453 | TAAGGACAGGGACATTTCCATCC CCGTGGAGTTAGGGTAGAGGA | 145 |
| SOD1 | NM_011434 | AACCAGTTGTGTTGTCAGGAC CCACCATGTTTCTTAGAGTGAGG | 139 |
| SOD2 | NM_013671 | GCGGTCGTGTAAACCTCAT CCAGAGCCTCGTGGTACTTC | 240 |
| SOD3 | NM_011435 | CTGAGGACTTCCCAGTGAC GGTGAGGGTGTCAGAGTGT | 195 |
| Group of Animals | Change in the Mass of Mouse Kidneys Relative to the Intact Group (%) | |
|---|---|---|
| 24 h | 72 h | |
| Intact control | 100 | 100 |
| I/R | 150 ± 10 * | 90 ± 10 * |
| I/R + Prx1 | 130 ± 10 *,# | 110 ± 10 # |
| I/R + Prx2 | 140 ± 10 * | 120 ± 10 # |
| Parameter | Intact Control | I/R | I/R + Prx1 | I/R + Prx2 | ||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | |
| Widening of the Bowman’s capsule | − | − | + | ++ | + | + | + | ++ |
| Congestion of the interstitium | − | − | ++ | ++ | ++ | ++ | ++ | ++ |
| Interstitial infiltration | − | − | + | + | + | + | + | + |
| Vessel congestion | − | − | ++ | ++ | + | + | ++ | + |
| Degeneration of convoluted tubules | − | − | ++ | +++ | + | + | ++ | + |
| Degeneration of straight tubules | − | − | + | + | + | - | + | + |
| Widening of convoluted tubules | − | − | + | + | + | - | + | + |
| Desquamation of epithelial cells | − | − | ++ | +++ | + | + | ++ | + |
| Disruption of epithelial cells | − | − | +++ | +++ | + | - | + | + |
| Group of Animals | Urea Concentration, mg/dL | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Intact control | 57 ± 9 | 54 ± 7 | 56 ± 8 |
| I/R | 290 ± 40 * | 125 ± 10 * | 80 ± 10 |
| I/R + Prx1 | 170 ± 40 *,# | 110 ± 20 * | 80 ± 10 |
| I/R + Prx2 | 240 ± 30 *,# | 120 ± 20 * | 75 ± 10 |
| Group of Animals | Creatinine Concentration, mg/dL | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Intact control | 0.28 ± 0.02 | 0.28 ± 0.02 | 0.28 ± 0.02 |
| I/R | 1.8 ± 0.2 * | 1.1 ± 0.3 * | 0.7 ± 0.2 * |
| I/R + Prx1 | 1.1 ± 0.2 *,# | 0.7 ± 0.1 *,# | 0.6 ± 0.1 * |
| I/R + Prx2 | 1.5 ± 0.2 *,# | 0.9 ± 0.2 * | 0.7 ± 0.1 * |
| Gene | Intact Control | I/R | I/R + Prx1 | I/R + Prx2 |
|---|---|---|---|---|
| KIM-1 | 1.0 | 106 ± 30 * | 71 ± 10 *,# | 85 ± 15 *,# |
| NRF-2 | 1.0 | 7.5 ± 0.8 * | 0.5 ± 0.2 # | 4.0 ± 0.8 *,# |
| NF-kB | 1.0 | 5.5 ± 0.7 * | 1.5 ± 0.3 # | 4.0 ± 0.9 * |
| IL-6 | 1.0 | 5.0 ± 0.8 * | 2.0 ± 0.5 # | 2.5 ± 1.0 # |
| IL-18 | 1.0 | 7.0 ± 1.0 * | 0.8 ± 0.2 # | 0.8 ± 0.1 # |
| iNOS | 1.0 | 14 ± 3.0 * | 1.3 ± 0.1 # | 3.5 ± 0.8 *,# |
| eNOS | 1.0 | 22.0 ± 4.0 * | 2.1 ± 0.5 # | 4.0 ± 1.0 *,# |
| AP-1 | 1.0 | 7.5 ± 0.8 * | 0.5 ± 0.2 # | 4.5 ± 0.2 *,# |
| Caspase-3 | 1.0 | 4.5 ± 0.7 * | 0.9 ± 0.3 # | 3.5 ± 0.8 * |
| CAT | 1.0 | 2.5 ± 0.3 | 1.1 ± 0.2 | 1.2 ± 0.2 |
| PRDX1 | 1.0 | 4.6 ± 0.5 * | 1.8 ± 0.2 # | 2.2 ± 0.3 *,# |
| PRDX2 | 1.0 | 3.8 ± 0.4 * | 1.1 ± 0.2 # | 1.3 ± 0.5 # |
| PRDX3 | 1.0 | 2.5 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.4 |
| PRDX4 | 1.0 | 2.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.3 |
| PRDX5 | 1.0 | 1.5 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.2 |
| PRDX6 | 1.0 | 4.2 ± 0.3 * | 2.0 ± 0.5 # | 2.3 ± 0.2 *,# |
| SOD1 | 1.0 | 5.3 ± 0.4 * | 1.9 ± 0.3 # | 2.1 ± 0.5 # |
| SOD2 | 1.0 | 8.5 ± 1.1 * | 2.9 ± 0.4 *,# | 3.5 ± 0.4 *,# |
| SOD3 | 1.0 | 2.8 ± 0.3 * | 2.0 ± 0.5 | 2.0 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharapov, M.G.; Goncharov, R.G.; Filkov, G.I.; Trofimenko, A.V.; Boyarintsev, V.V.; Novoselov, V.I. Comparative Study of Protective Action of Exogenous 2-Cys Peroxiredoxins (Prx1 and Prx2) Under Renal Ischemia-Reperfusion Injury. Antioxidants 2020, 9, 680. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080680
Sharapov MG, Goncharov RG, Filkov GI, Trofimenko AV, Boyarintsev VV, Novoselov VI. Comparative Study of Protective Action of Exogenous 2-Cys Peroxiredoxins (Prx1 and Prx2) Under Renal Ischemia-Reperfusion Injury. Antioxidants. 2020; 9(8):680. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080680
Chicago/Turabian StyleSharapov, Mars G., Ruslan G. Goncharov, Gleb I. Filkov, Alexander V. Trofimenko, Valery V. Boyarintsev, and Vladimir I. Novoselov. 2020. "Comparative Study of Protective Action of Exogenous 2-Cys Peroxiredoxins (Prx1 and Prx2) Under Renal Ischemia-Reperfusion Injury" Antioxidants 9, no. 8: 680. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox9080680