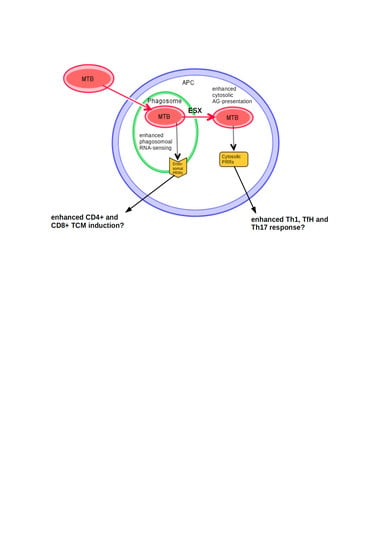
RNA Sensing of Mycobacterium tuberculosis and Its Impact on TB Vaccination Strategies
Abstract
:1. Introduction
2. RNA Sensing and Activation of APCs
2.1. Endosomal Mycobacterial RNA Sensing
2.1.1. TLR8—The Most Prominent Phagosomal RNA Sensor
2.1.2. TLR7—ssRNA Sensor in pDCs
2.1.3. TLR3—Recognizing dsRNA
2.2. Cytosolic Mycobacterial RNA Sensing
2.2.1. NLRP3—The Cytosolic Multisensor
2.2.2. Other Cytosolic Sensors
2.3. Synergy of Different PRRs
3. Downstream Pathways and T-Cell Activation after Mycobacterial RNA Sensing
3.1. IL-12—The Key Cytokine for a Th1/17 Response
3.2. IL-21 and the Significance of TfH Cells in TB
3.3. Type I Interferons and Their Potential Rediscovered Importance in Early TB Immunity
4. The Impact of RNA Sensing on Vaccination
4.1. Using Potential Effects of Endosomal RNA Sensing to Generate New Vaccines
4.2. Canonical BCG and Its Potential Improvement through Cytosolic RNA Sensing
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| BMDM | Bone marrow-derived macrophages |
| BMP | Bone morphogenesis protein |
| CFP-10 | Culture filtrate protein 10 |
| cGAS | Cyclic GMP-AMP synthase |
| CXCR5 | CXC-motif-chemokine receptor 5 |
| CYBB | Cytochrome b (-245) beta |
| DAMP | Danger-associated molecular pattern |
| DC | Dendritic cell |
| DCIR | C-type II lectin dendritic cell immunoreceptor |
| EGFR | Epidermal growth factor receptor |
| EPTB | Extrapulmonary TB |
| ESAT-6 | 6 kDa early secretory antigenic target |
| ESX-1 | ESAT-6 secretion system 1 |
| HMBPP | (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate |
| IFN | Interferon |
| IL1RA | The Interleukin-1 receptor antagonist |
| IL12RB2 | IL-12 receptor subunit beta 2 |
| IL | Interleukin |
| IRF | Interferon response factor |
| LGP2 | Laboratory of genetics and physiology 2 |
| MAMP | Microbe-associated molecular patterns |
| MDA5 | Melanoma-differentiated gene 5 |
| mDC | Myeloid dendritic cell |
| moDC | Monocyte-derived dendritic cell |
| MTB | Mycobacterium tuberculosis |
| MyD88 | Myeloid differentiation primary response 88 |
| NEMO | NF-kappa-B essential modulator |
| NK | Natural killer |
| NLRP3 | Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 |
| NOX2 | NADPH oxidase 2 |
| NRL | Nod-like receptor |
| OAS | Oligoadenylate synthetases |
| OASL | 2′-5′Oligoadenylate synthases-like |
| PAMP | Pattern associated molecular pattern |
| PBMC | Peripheral blood mononuclear cell |
| PD1 | Programmed cell death protein 1 |
| pDC | Plasmatic dendritic cell |
| PKR | Protein kinase R |
| PRR | Pattern recognition receptors |
| PTB | Pulmonary TB |
| RAGE | Receptor for advanced glycation end product |
| rBCG | Recombinant BCG |
| RD-1 | Region of deletion 1 |
| RIG-I | Retinoic acid inducible gene I |
| RLR | Rig-I-like Receptor |
| ROS | Radical oxygen species |
| STING | Stimulator of interferon genes |
| TB | Tuberculosis |
| TgF | Transforming growth factor |
| TLR | Toll-like receptor |
| TNFα | Tumor necrosis factor alpha |
| TRAF | TNF receptor associated factor |
| TRIF | TIR-domain-containing adapter-inducing interferon-β. |
References
- Barnighausen, T.; Bloom, D.E.; Cafiero-Fonseca, E.T.; O’Brien, J.C. Valuing vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12313–12319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Rivera, A.; Siracusa, M.C.; Yap, G.S.; Gause, W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 2016, 17, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, C.; Bowie, A.G. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013, 21, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashiro, L.H.; Oliveira, S.C.; Báfica, A. Innate immune sensing of nucleic acids from mycobacteria. Mcrobes Infect. 2014, 16, 991–997. [Google Scholar] [CrossRef]
- Reiling, N.; Homolka, S.; Kohl, T.A.; Steinhäuser, C.; Kolbe, K.; Schütze, S.; Brandenburg, J. Shaping the niche in macrophages: Genetic diversity of the M. tuberculosis complex and its consequences for the infected host. Int. J. Med. Microbiol. 2018, 308, 118–128. [Google Scholar] [CrossRef]
- Ugolini, M.; Gerhard, J.; Burkert, S.; Jensen, K.J.; Georg, P.; Ebner, F.; Volkers, S.M.; Thada, S.; Dietert, K.; Bauer, L.; et al. Recognition of microbial viability via TLR8 drives TFHcell differentiation and vaccine responses. Nat. Immunol. 2018, 19, 386–396. [Google Scholar] [CrossRef]
- Castillo, E.F.; Dekonenko, A.; Arko-Mensah, J.; Mandell, M.A.; Dupont, N.; Jiang, S.; Delgado-Vargas, M.; Timmins, G.S.; Bhattacharya, D.; Yang, H.; et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, E3168–E3176. [Google Scholar] [CrossRef] [Green Version]
- Padhi, A.; Pattnaik, K.; Biswas, M.; Jagadeb, M.; Behera, A.; Sonawane, A. Mycobacterium tuberculosis LprE Suppresses TLR2-Dependent Cathelicidin and Autophagy Expression to Enhance Bacterial Survival in Macrophages. J. Immunol. 2019, 203, 2665–2678. [Google Scholar] [CrossRef]
- De Marcken, M.; Dhaliwal, K.; Danielsen, A.C.; Gautron, A.S.; Dominguez-Villar, M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci. Signal. 2019, 12, 1347. [Google Scholar] [CrossRef]
- Cervantes, J.L.; La Vake, C.J.; Weinerman, B.; Luu, S.; O’connell, C.; Verardi, P.H.; Salazar, J.C. Human TLR8 is activated upon recognition of Borrelia burgdorferi RNA in the phagosome of human monocytes. J. Leukoc. Biol. 2013, 94, 1231–1241. [Google Scholar] [CrossRef] [Green Version]
- Ohto, U.; Tanji, H.; Shimizu, T. Structure and function of toll-like receptor 8. Microbes Infect. 2014, 16, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Moen, S.H.; Ehrnström, B.; Kojen, J.F.; Yurchenko, M.; Beckwith, K.S.; Afset, J.E.; Damås, J.K.; Hu, Z.; Yin, H.; Espevik, T.; et al. Human Toll-like Receptor 8 (TLR8) Is an Important Sensor of Pyogenic Bacteria, and Is Attenuated by Cell Surface TLR Signaling. Front. Immunol. 2019, 10, 1209. [Google Scholar] [CrossRef] [Green Version]
- Gidon, A.; Åsberg, S.E.; Louet, C.; Ryan, L.; Haug, M.; Flo, T.H. Persistent mycobacteria evade an antibacterial program mediated by phagolysosomal TLR7/8/MyD88 in human primary macrophages. PLoS Pathog. 2017, 13, e1006551. [Google Scholar]
- Awais, M.; Wang, K.; Lin, X.; Qian, W.; Zhang, N.; Wang, C.; Wang, K.; Zhao, L.; Fu, Z.F.; Cui, M. TLR7 deficiency leads to TLR8 compensative regulation of immune response against JEV in mice. Front. Immunol. 2017, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Sela, U.; Park, C.G.; Park, A.; Olds, P.; Wang, S.; Steinman, R.M.; Fischetti, V.A. Dendritic Cells Induce a Subpopulation of IL-12Rβ2-Expressing Treg that Specifically Consumes IL-12 to Control Th1 Responses. PLoS ONE 2016, 11, e0146412. [Google Scholar] [CrossRef] [Green Version]
- Demaria, O.; Pagni, P.P.; Traub, S.; de Gassart, A.; Branzk, N.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Flavell, R.A.; Alexopoulou, L. TLR8 deficiency leads to autoimmunity in mice. J. Clin. Investig. 2010, 120, 3651–3662. [Google Scholar] [CrossRef]
- Jordao, L.; Bleck, C.K.E.; Mayorga, L.; Griffiths, G.; Anes, E. On the killing of mycobacteria by macrophages. Cell. Microbiol. 2008, 10, 529–548. [Google Scholar] [CrossRef]
- Tang, J.; Sun, M.; Shi, G.; Xu, Y.; Han, Y.; Li, X.; Dong, W.; Zhan, L.; Qin, C. Toll-like receptor 8 agonist strengthens the protective efficacy of ESAT-6 immunization to Mycobacterium tuberculosis infection. Front. Immunol. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Zhan, L.; Qin, C. Microbial Pathogenesis Inhibition of TLR8 mediated signaling promotes BCG induced apoptosis in THP-1 cells. Microb. Pathog. 2016, 93, 78–82. [Google Scholar] [CrossRef]
- Ramos-Martinez, A.G.; Valtierra-Alvarado, M.A.; Garcia-Hernandez, M.H.; Hernandez-Pando, R.; Castañeda-Delgado, J.E.; Cougoule, C.; Rivas-Santiago, B.; Neyrolles, O.; Enciso-Moreno, J.A.; Lugo-Villarino, G.; et al. Variability in the virulence of specific Mycobacterium tuberculosis clinical isolates alters the capacity of human dendritic cells to signal for T cells. Memórias do Instituto Oswaldo Cruz 2019, 114, 190102. [Google Scholar] [CrossRef] [Green Version]
- Keegan, C.; Krutzik, S.; Schenk, M.; Scumpia, P.O.; Lu, J.; Pang, Y.L.J.; Russell, B.S.; Lim, K.S.; Shell, S.; Prestwich, E.; et al. Mycobacterium tuberculosis Transfer RNA Induces IL-12p70 via Synergistic Activation of Pattern Recognition Receptors within a Cell Network. J. Immunol. 2018, 200, 3244–3258. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.; Wang, J.; Wu, M.; Lai, H.; Chiang, B.; Yu, C. Interleukin 23/interleukin 17 axis activated by Mycobacterium avium complex (MAC) is attenuated in patients with MAC-lung disease. Tuberculosis 2018, 110, 7–14. [Google Scholar] [CrossRef]
- Tamassia, N.; Arruda-Silva, F.; Wright, H.L.; Moots, R.J.; Gardiman, E.; Bianchetto-Aguilera, F.; Gasperini, S.; Capone, M.; Maggi, L.; Annunziato, F.; et al. Human neutrophils activated via TLR8 promote Th17 polarization through IL-23. J. Leukoc. Biol. 2019, 105, 1155–1165. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Taube, S.; Hamouda, O.; Kücherer, C.; Poggensee, G.; Jessen, H.; Eckert, J.K.; Neumann, K.; Storek, A.; Pouliot, M.; et al. A functional toll-like receptor 8 variant is associated with HIV disease restriction. J. Infect. Dis. 2008, 198, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-H.; Eng, H.-L.; Lin, K.-H.; Liu, H.-C.; Chang, C.-H.; Lin, T.-M. Functional polymorphisms of TLR8 are associated with hepatitis C virus infection. Immunology 2014, 141, 540–548. [Google Scholar] [CrossRef]
- El-Bendary, M.; Neamatallah, M.; Elalfy, H.; Besheer, T.; Elkholi, A.; El-Diasty, M.; Elsareef, M.; Zahran, M.; El-Aarag, B.; Gomaa, A.; et al. The association of single nucleotide polymorphisms of Toll-like receptor 3, Toll-like receptor 7 and Toll-like receptor 8 genes with the susceptibility to HCV infection. Br. J. Biomed. Sci. 2018, 75, 175–181. [Google Scholar] [CrossRef]
- Davila, S.; Hibberd, M.L.; Hari Dass, R.; Wong, H.E.E.; Sahiratmadja, E.; Bonnard, C.; Alisjahbana, B.; Szeszko, J.S.; Balabanova, Y.; Drobniewski, F.; et al. Genetic Association and Expression Studies Indicate a Role of Toll-Like Receptor 8 in Pulmonary Tuberculosis. PLoS Genet. 2008, 4, e1000218. [Google Scholar] [CrossRef] [Green Version]
- Varzari, A.; Deyneko, I.V.; Vladei, I.; Grallert, H.; Schieck, M.; Tudor, E.; Illig, T. Genetic variation in TLR pathway and the risk of pulmonary tuberculosis in a Moldavian population. Infect. Genet. Evol. 2019, 68, 84–90. [Google Scholar] [CrossRef]
- Dalgic, N.; Tekin, D.; Kayaalti, Z.; Cakir, E.; Soylemezoglu, T.; Sancar, M. Relationship between toll-like receptor 8 gene polymorphisms and pediatric pulmonary tuberculosis. Dis. Markers 2011, 31, 33–38. [Google Scholar] [CrossRef]
- Selvaraj, P.; Harishankar, M.; Singh, B.; Jawahar, M.S.; Banurekha, V.V. Toll-like receptor and TIRAP gene polymorphisms in pulmonary tuberculosis patients of South India. Tuberculosis 2010, 90, 306–310. [Google Scholar] [CrossRef]
- Lai, Y.F.; Lin, T.M.; Wang, C.H.; Su, P.Y.; Wu, J.T.; Lin, M.C.; Eng, H.L. Functional polymorphisms of the TLR7 and TLR8 genes contribute to Mycobacterium tuberculosis infection. Tuberculosis 2016, 98, 125–131. [Google Scholar] [CrossRef]
- Bukhari, M.; Aslam, M.A.; Khan, A.; Iram, Q.; Akbar, A.; Naz, A.G.; Ahmad, S.; Ahmad, M.M.; Ashfaq, U.A.; Aziz, H.; et al. TLR8 gene polymorphism and association in bacterial load in southern Punjab of Pakistan: An association study with pulmonary tuberculosis. Int. J. Immunogenet. 2015, 42, 46–51. [Google Scholar] [CrossRef]
- Wang, M.-G.; Zhang, M.-M.; Wang, Y.; Wu, S.-Q.; Zhang, M.; He, J.-Q. Association of TLR8 and TLR9 polymorphisms with tuberculosis in a Chinese Han population: A case-control study. BMC Infect. Dis. 2018, 18, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [Green Version]
- Eng, H.; Hsu, Y.; Lin, T. Differences in TLR7/8 activation between monocytes and macrophages. Biochem. Biophys. Res. Commun. 2018, 497, 319–325. [Google Scholar] [CrossRef]
- Bao, M.; Yi, Z.; Fu, Y. Activation of TLR7 Inhibition of Mycobacterium Tuberculosis Survival by Autophagy in RAW 264.7 Macrophages. J. Cell. Biochem. 2017, 118, 4222–4229. [Google Scholar] [CrossRef]
- Matsumoto, M.; Seya, T. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef]
- Mvubu, N.E.; Pillay, B.; McKinnon, L.R.; Pillay, M. Mycobacterium tuberculosis strains induce strain-specific cytokine and chemokine response in pulmonary epithelial cells. Cytokine 2018, 104, 53–64. [Google Scholar] [CrossRef]
- Bai, W.; Liu, H.; Ji, Q.; Zhou, Y.; Liang, L.; Zheng, R.; Chen, J. TLR3 regulates mycobacterial RNA-induced IL-10 production through the PI3K/AKT signaling pathway. Cell. Signal. 2014, 26, 942–950. [Google Scholar] [CrossRef]
- Mahadik, K.; Prakhar, P.; Rajmani, R.S.; Singh, A.; Balaji, K.N. c-Abl-TWIST1 epigenetically dysregulate inflammatory responses during mycobacterial infection by co-regulating bone morphogenesis protein and miR27a. Front. Immunol. 2018, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Napier, R.J.; Rafi, W.; Cheruvu, M.; Powell, K.R.; Zaunbrecher, M.A.; Bornmann, W.; Salgame, P.; Shinnick, T.M.; Kalman, D. Imatinib-Sensitive Tyrosine Kinases Regulate Mycobacterial Pathogenesis and Represent Therapeutic Targets against Tuberculosis. Cell Host Microbe 2011, 10, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Bertheloot, D.; Naumovski, A.L.; Langhoff, P.; Horvath, G.L.; Jin, T.; Xiao, T.S.; Garbi, N.; Agrawal, S.; Kolbeck, R.; Latz, E. RAGE Enhances TLR Responses through Binding and Internalization of RNA. J. Immunol. 2016, 197, 4118–4126. [Google Scholar] [CrossRef] [Green Version]
- Lui, G.; Wong, C.K.; Ip, M.; Chu, Y.J.; Yung, I.M.H.; Cheung, C.S.K.; Zheng, L.; Lam, J.S.Y.; Wong, K.T.; Sin, W.W.Y.; et al. HMGB1/RAGE signaling and pro-inflammatory cytokine responses in non-HIV adults with active pulmonary tuberculosis. PLoS ONE 2016, 11, e0159132. [Google Scholar] [CrossRef]
- Da Silva, L.F.; Skupien, E.C.; Lazzari, T.K.; Holler, S.R.; de Almeida, E.G.C.; Zampieri, L.R.; Coutinho, S.E.; Andrades, M.; Silva, D.R. Advanced glycation end products (AGE) and receptor for AGE (RAGE) in patients with active tuberculosis, and their relationship between food intake and nutritional status. PLoS ONE 2019, 14, e0213991. [Google Scholar] [CrossRef]
- Houben, D.; Demangel, C.; van Ingen, J.; Perez, J.; Baldeón, L.; Abdallah, A.M.; Caleechurn, L.; Bottai, D.; van Zon, M.; de Punder, K.; et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell. Microbiol. 2012, 14, 1287–1298. [Google Scholar] [CrossRef]
- Ray, S.; Vazquez Reyes, S.; Xiao, C.; Sun, J. Effects of membrane lipid composition on Mycobacterium tuberculosis EsxA membrane insertion: A dual play of fluidity and charge. Tuberculosis 2019, 118, 101854. [Google Scholar] [CrossRef]
- Basu, S.; Fowler, B.J.; Kerur, N.; Arnvig, K.B.; Rao, N.A. NLRP3 inflammasome activation by mycobacterial ESAT-6 and dsRNA in intraocular tuberculosis. Microb. Pathog. 2018, 114, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Sander, L.E.; Davis, M.J.; Boekschoten, M.V.; Amsen, D.; Dascher, C.C.; Ryffel, B.; Swanson, J.A.; Müller, M.; Blander, J.M. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 2011, 474, 385–389. [Google Scholar] [CrossRef]
- Barbet, G.; Sander, L.E.; Geswell, M.; Leonardi, I.; Cerutti, A.; Iliev, I.; Blander, J.M. Sensing Microbial Viability through Bacterial RNA Augments T Follicular Helper Cell and Antibody Responses. Immunity 2018, 48, 584–598.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, E.P.; Riteau, N.; Moayeri, M.; Maier, N.; Mayer-Barber, K.D.; Pereira, R.M.; Lage, S.L.; Kubler, A.; Bishai, W.R.; Andrade, B.B.; et al. Lysosomal Cathepsin Release Is Required for NLRP3-Inflammasome Activation by Mycobacterium tuberculosis in Infected Macrophages. Front. Immunol. 2018, 9, 1427. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Mizumo, S.; Horai, R.; Iwakura, Y.; Sugawara, I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab. Investig. 2000, 80, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eklund, D.; Welin, A.; Andersson, H.; Verma, D.; Söderkvist, P.; Stendahl, O.; Särndahl, E.; Lerm, M. Human Gene Variants Linked to Enhanced NLRP3 Activity Limit Intramacrophage Growth of Mycobacterium tuberculosis. J. Infect. Dis. 2014, 209, 749–753. [Google Scholar] [CrossRef] [PubMed]
- De Lima, D.S.; Ogusku, M.M.; Sadahiro, A.; Pontillo, A. Infection, Genetics and Evolution In fl ammasome genetics contributes to the development and control of active pulmonary tuberculosis. Infect. Genet. Evol. 2016, 41, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Abate, E.; Blomgran, R.; Verma, D.; Lerm, M.; Fredrikson, M.; Belayneh, M.; Söderkvist, P.; Stendahl, O.; Schön, T. Polymorphisms in CARD8 and NLRP3 are associated with extrapulmonary TB and poor clinical outcome in active TB in Ethiopia. Sci. Rep. 2019, 9, 3126. [Google Scholar] [CrossRef]
- Ravimohan, S.; Nfanyana, K.; Tamuhla, N.; Tiemessen, C.T.; Weissman, D.; Bisson, G.P. Common Variation in NLRP3 Is Associated With Early Death and Elevated Inflammasome Biomarkers among Advanced HIV/TB Co-infected Patients in Botswana. Open Forum Infect. Dis. 2018, 5, ofy075. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [Green Version]
- Andreu, N.; Phelan, J.; de Sessions, P.F.; Cliff, J.M.; Clark, T.G.; Hibberd, M.L. Primary macrophages and J774 cells respond differently to infection with Mycobacterium tuberculosis. Sci. Rep. 2017, 7, 42225. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, S.; Haridas, V.; Nambu, A.; Jasenosky, L.D.; Sadhukhan, S.; Ebert, T.S.; Hornung, V.; Gail, H.C.; Falvo, J.; Goldfeld, A.E. Cytoplasmic RNA Sensor Pathways and Nitazoxanide Broadly Inhibit Intracellular Mycobacterium tuberculosis Growth. iScience 2019, 22, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Schorey, J.S. Mycobacterium tuberculosis –induced IFN-β production requires cytosolic DNA and RNA sensing pathways. J. Exp. Med. 2018, 215, 2919–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisien, J.; Lenoir, J.J.; Mandhana, R.; Rodriguez, K.R.; Qian, K.; Bruns, A.M.; Horvath, C.M. RNA sensor LGP2 inhibits TRAF ubiquitin ligase to negatively regulate innate immune signaling. EMBO Rep. 2018, 19, e45176. [Google Scholar] [CrossRef] [PubMed]
- Leisching, G.; Wiid, I.; Baker, B. The Association of OASL and Type I Interferons in the Pathogenesis and Survival of Intracellular Replicating Bacterial Species. Front. Cell. Infect. Microbiol. 2017, 7, 3–8. [Google Scholar] [CrossRef] [Green Version]
- De Toledo-Pinto, T.G.; Ferreira, A.B.R.; Ribeiro-Alves, M.; Rodrigues, L.S.; Batista-Silva, L.R.; de Silva, B.J.A.; Lemes, R.M.R.; Martinez, A.N.; Sandoval, F.G.; Alvarado-Arnez, L.E.; et al. STING-Dependent 2′-5′ Oligoadenylate Synthetase-Like Production Is Required for Intracellular Mycobacterium leprae Survival. J. Infect. Dis. 2016, 214, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundhra, S.; Bryk, R.; Hawryluk, N.; Zhang, T.; Jiang, X.; Nathan, C.F. Evidence for dispensability of protein kinase R in host control of tuberculosis. Eur. J. Immunol. 2018, 48, 612–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, F.E.; Chang, K.; Minoda, Y.; Rojas, I.M.L.; Haigh, O.L.; Daraj, G.; Tullett, K.M.; Radford, K.J. Activation of human CD141 + and CD1c + dendritic cells in vivo with combined TLR3 and TLR7/8 ligation. Immunol. Cell Biol. 2018, 96, 390–400. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Tamjidi, S.; Nourishirazi, E.; Guinet, E. TLR8 combined withTLR3 or TLR4 agonists enhances DC-NK driven effector Tc1 cells. Immunol. Lett. 2018, 193, 58–66. [Google Scholar] [CrossRef]
- Speth, M.T.; Repnik, U.; Müller, E.; Spanier, J.; Kalinke, U.; Corthay, A.; Griffiths, G. Poly(I:C)-Encapsulating Nanoparticles Enhance Innate Immune Responses to the Tuberculosis Vaccine Bacille Calmette–Guérin (BCG) via Synergistic Activation of Innate Immune Receptors. Mol. Pharm. 2017, 14, 4098–4112. [Google Scholar] [CrossRef]
- Bösl, K.; Giambelluca, M.; Haug, M.; Bugge, M.; Espevik, T.; Kandasamy, R.K.; Bergstrøm, B. Coactivation of TLR2 and TLR8 in Primary Human Monocytes Triggers a Distinct Inflammatory Signaling Response. Front. Physiol. 2018, 9, 618. [Google Scholar] [CrossRef]
- Vierbuchen, T.; Bang, C.; Rosigkeit, H.; Schmitz, R.A.; Heine, H. The Human-Associated Archaeon Methanosphaera stadtmanae is recognized through its RNA and Induces TLR8-Dependent NLRP3 Inflammasome Activation. Front. Immunol. 2017, 8, 1535. [Google Scholar] [CrossRef] [Green Version]
- Dietsch, G.N.; Lu, H.; Yang, Y.; Morishima, C.; Chow, L.Q.; Disis, M.L.; Hershberg, R.M. Coordinated Activation of Toll-Like Receptor8 (TLR8) and NLRP3 by the TLR8 Agonist, VTX-2337, Ignites Tumoricidal Natural Killer Cell Activity. PLoS ONE 2016, 11, e0148764. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Gao, J.; Taxman, D.J.; Ting, J.P.Y.; Su, L. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J. Biol. Chem. 2014, 289, 21716–21726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makni-Maalej, K.; Marzaioli, V.; Boussetta, T.; Belambri, S.A.; Gougerot-Pocidalo, M.-A.; Hurtado-Nedelec, M.; Dang, P.M.; El-Benna, J. TLR8, but not TLR7, induces the priming of the NADPH oxidase activation in human neutrophils. J. Leukoc. Biol. 2015, 97, 1081–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruchard, M.; Mignot, G.; Derangère, V.; Chalmin, F.; Chevriaux, A.; Végran, F.; Boireau, W.; Simon, B.; Ryffel, B.; Connat, J.L.; et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 2013, 19, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sokolovska, A.; Becker, C.E.; Ip, W.K.E.; Rathinam, V.A.K.; Brudner, M.; Paquette, N.; Tanne, A.; Vanaja, S.K.; Moore, K.J.; Fitzgerald, K.A.; et al. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat. Immunol. 2013, 14, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Skogmar, S.; Schön, T.; Balcha, T.T.; Jemal, Z.H.; Tibesso, G.; Björk, J.; Björkman, P. CD4 cell levels during treatment for tuberculosis (TB) in Ethiopian adults and clinical markers associated with CD4 lymphocytopenia. PLoS ONE 2013, 8, 6–12. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, G.; Chen, X. Th1 cytokines, true functional signatures for protective immunity against TB? Cell. Mol. Immunol. 2018, 15, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Khader, S.A.; Partida-Sanchez, S.; Bell, G.; Jelley-Gibbs, D.M.; Swain, S.; Pearl, J.E.; Ghilardi, N.; DeSauvage, F.J.; Lund, F.E.; Cooper, A.M. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J. Exp. Med. 2006, 203, 1805–1815. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, J.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.-L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef] [Green Version]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S. Human Th17 cells: Are they different from murine Th17 cells? Eur. J. Immunol. 2009, 39, 637–640. [Google Scholar] [CrossRef]
- Stein, T.; Wollschlegel, A.; Te, H.; Weiss, J.; Joshi, K.; Kinzel, B.; Billich, A.; Guntermann, C.; Lehmann, J.C.U. Interferon regulatory factor 5 and nuclear factor kappa-B exhibit cooperating but also divergent roles in the regulation of pro-inflammatory cytokines important for the development of TH1 and TH17 responses. FEBS J. 2018, 285, 3097–3113. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Chen, Z.W. The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell. Mol. Immunol. 2018, 15, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redford, P.S.; Boonstra, A.; Read, S.; Pitt, J.; Graham, C.; Stavropoulos, E.; Bancroft, G.J.; O’Garra, A. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur. J. Immunol. 2010, 40, 2200–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Zhang, M.; Yan, B.; Zhang, K.; Chen, M.; Deng, S. Imbalance of Th17 and Treg in peripheral blood mononuclear cells of active tuberculosis patients. Braz. J. Infect. Dis. 2017, 21, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cui, G.; Jia, H.; Zhu, Y.; Ding, Y.; Chen, J.; Lu, C.; Ye, P.; Gao, H.; Li, L.; et al. Decreased IL-17 during treatment of sputum smear-positive pulmonary tuberculosis due to increased regulatory T cells and IL-10. J. Transl. Med. 2016, 14, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulter, F.; Parrish, A.; Manning, D.; Kampmann, B.; Mendy, J.; Garand, M.; Lewinsohn, D.M.; Riley, E.M.; Sutherland, J.S. IL-17 Production from T Helper 17, Mucosal-Associated Invariant T, and γδ Cells in Tuberculosis Infection and Disease. Front. Immunol. 2017, 8, 1252. [Google Scholar] [CrossRef] [Green Version]
- Gopal, R.; Lin, Y.; Obermajer, N.; Slight, S.; Nuthalapati, N.; Ahmed, M.; Kalinski, P.; Khader, S.A. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immnol. 2012, 42, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Milano, M.; Moraes, M.O.; Rodenbusch, R.; Carvalho, C.X.; Delcroix, M.; Mousquer, G.; Da Costa, L.L.; Unis, G.; Costa, E.R.D.; Rossetti, M.L.R. Single nucleotide polymorphisms in IL17A and IL6 are associated with decreased risk for pulmonary tuberculosis in southern Brazilian population. PLoS ONE 2016, 11, e0147814. [Google Scholar] [CrossRef] [Green Version]
- Eskandari-Nasab, E.; Moghadampour, M.; Tahmasebi, A.; Asadi-Saghandi, A. Interleukin-17 A and F gene polymorphisms affect the risk of tuberculosis: An updated meta-analysis. Indian J. Tuberc. 2018, 65, 200–207. [Google Scholar] [CrossRef]
- Yang, R.; Yao, L.; Shen, L.; Sha, W.; Modlin, R.L.; Shen, H.; Chen, Z.W. IL-12 Expands and Differentiates Human Vγ2Vδ2 T Effector Cells Producing Antimicrobial Cytokines and Inhibiting Intracellular Mycobacterial Growth. Front. Immunol. 2019, 10, 1742. [Google Scholar] [CrossRef]
- Shen, L.; Frencher, J.; Huang, D.; Wang, W.; Yang, E.; Chen, C.Y.; Zhang, Z.; Wang, R.; Qaqish, A.; Larsen, M.H.; et al. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc. Natl. Acad. Sci. USA 2019, 116, 6371–6378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booty, M.G.; Barreira-Silva, P.; Carpenter, S.M.; Nunes-Alves, C.; Jacques, M.K.; Stowell, B.L.; Jayaraman, P.; Beamer, G.; Behar, S.M. IL-21 signaling is essential for optimal host resistance against Mycobacterium tuberculosis infection. Sci. Rep. 2016, 6, 36720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheekatla, S.S.; Tripathi, D.; Venkatasubramanian, S.; Paidipally, P.; Welch, E.; Tvinnereim, A.R.; Nurieva, R.; Vankayalapati, R. IL-21 Receptor Signaling Is Essential for Optimal CD4 + T Cell Function and Control of Mycobacterium tuberculosis Infection in Mice. J. Immunol. 2017, 199, 2815–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moguche, A.O.; Shafiani, S.; Clemons, C.; Larson, R.P.; Dinh, C.; Higdon, L.E.; Cambier, C.J.; Sissons, J.R.; Gallegos, A.M.; Fink, P.J.; et al. ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis. J. Exp. Med. 2015, 212, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, K.; Spolski, R.; Ettinger, R.; Kim, H.-P.; Wang, G.; Qi, C.-F.; Hwu, P.; Shaffer, D.J.; Akilesh, S.; Roopenian, D.C.; et al. Regulation of B Cell Differentiation and Plasma Cell Generation by IL-21, a Novel Inducer of Blimp-1 and Bcl-6. J. Immunol. 2004, 173, 5361–5371. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, X.; Wang, B.; Fu, L.; Liu, G.; Lu, Y.; Cao, M.; Huang, H.; Javid, B. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2017, 114, 5023–5028. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, N.; Thormann, V.; Hu, B.; Köhler, A.; Imai-Matsushima, A.; Locht, C.; Arnett, E.; Schlesinger, L.S.; Zoller, T.; Schürmann, M.; et al. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol. Med. 2016, 8, 1325–1339. [Google Scholar] [CrossRef]
- Achkar, J.M.; Chan, J.; Casadevall, A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol. Rev. 2015, 264, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Tran, A.C.; Kim, M.-Y.; Reljic, R. Emerging Themes for the Role of Antibodies in Tuberculosis. Immune Netw. 2019, 19, e24. [Google Scholar] [CrossRef]
- Tanner, R.; Villarreal-Ramos, B.; Vordermeier, H.M.; McShane, H. The Humoral Immune Response to BCG Vaccination. Front. Immunol. 2019, 10, 1317. [Google Scholar] [CrossRef] [Green Version]
- Kozakiewicz, L.; Chen, Y.; Xu, J.; Wang, Y.; Dunussi-Joannopoulos, K.; Ou, Q.; Flynn, J.L.; Porcelli, S.A.; Jacobs, W.R.; Chan, J. B Cells Regulate Neutrophilia during Mycobacterium tuberculosis Infection and BCG Vaccination by Modulating the Interleukin-17 Response. PLoS Pathog. 2013, 9, e1003472. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, A.; Kisielow, J.; Schmitz, I.; Freigang, S.; Shamshiev, A.T.; Weber, J.; Marsland, B.J.; Oxenius, A.; Kopf, M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 2009, 324, 1576–1580. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, N.; Nakamura, R.; Hatano, S.; Yamada, H.; Sun, X.; Ohara, N.; Yoshikai, Y. Interleukin-21 Induces Short-Lived Effector CD8+ T Cells but Does Not Inhibit Their Exhaustion after Mycobacterium bovis BCG Infection in Mice. Infect. Immun. 2018, 86, e00147-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paidipally, P.; Tripathi, D.; Van, A.; Radhakrishnan, R.K.; Dhiman, R.; Venkatasubramanian, S.; Devalraju, K.P.; Tvinnereim, A.R.; Valluri, V.L.; Vankayalapati, R. Interleukin-21 Regulates Natural Killer Cell Responses During Mycobacterium tuberculosis Infection. J. Infect. Dis. 2018, 217, 1323–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatasubramanian, S.; Cheekatla, S.; Paidipally, P.; Tripathi, D.; Welch, E.; Tvinnereim, A.R.; Nurieva, R.; Vankayalapati, R. IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mycobacterium tuberculosis. Mucosal Immunol. 2017, 10, 1031–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNab, F.W.; Ewbank, J.; Howes, A.; Moreira-Teixeira, L.; Martirosyan, A.; Ghilardi, N.; Saraiva, M.; O’Garra, A. Type I IFN Induces IL-10 Production in an IL-27–Independent Manner and Blocks Responsiveness to IFN-γ for Production of IL-12 and Bacterial Killing in Mycobacterium tuberculosis –Infected Macrophages. J. Immunol. 2014, 193, 3600–3612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troegeler, A.; Mercier, I.; Cougoule, C.; Pietretti, D.; Colom, A.; Duval, C.; Vu Manh, T.P.; Capilla, F.; Poincloux, R.; Pingris, K.; et al. C-type lectin receptor DCIR modulates immunity to tuberculosis by sustaining type I interferon signaling in dendritic cells. Proc. Natl. Acad. Sci. USA 2017, 114, E540–E549. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Teixeira, L.; Sousa, J.; McNab, F.W.; Torrado, E.; Cardoso, F.; Machado, H.; Castro, F.; Cardoso, V.; Gaifem, J.; Wu, X.; et al. Type I IFN Inhibits Alternative Macrophage Activation during Mycobacterium tuberculosis Infection and Leads to Enhanced Protection in the Absence of IFN-γ Signaling. J. Immunol. 2016, 197, 4714–4726. [Google Scholar] [CrossRef] [Green Version]
- Parlato, S.; Chiacchio, T.; Salerno, D.; Petrone, L.; Castiello, L.; Romagnoli, G.; Canini, I.; Goletti, D.; Gabriele, L. Impaired IFN-α-mediated signal in dendritic cells differentiates active from latent tuberculosis. PLoS ONE 2018, 13, e0189477. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Sun, Y.; He, C.; Qiu, X.; Zhou, D.; Ye, Z.; Long, Y.; Tang, T.; Su, X.; Ma, J. The immune characterization of interferon-β responses in tuberculosis patients. Microbiol. Immunol. 2018, 62, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Sable, S.B.; Posey, J.E.; Scriba, T.J. Tuberculosis Vaccine Development: Progress in Clinical Evaluation. Clin. Microbiol. Rev. 2019, 33, e00100-19. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.-E.; Ahn, J.-H.; Min, S.; Kim, H.; Seo, J.; Yeo, S.-G.; Ko, H.-J. Development of New Preventive and Therapeutic Vaccines for Tuberculosis. Immune Netw. 2018, 18, e17. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E.; Kaufmann, S.H.E. Next-Generation Vaccines Based on Bacille Calmette-Guérin. Front. Immunol. 2018, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Ottenhoff, T.H.M.; Kaufmann, S.H.E. Vaccines against Tuberculosis: Where Are We and Where Do We Need to Go? PLoS Pathog. 2012, 8, e1002607. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E. Neue Impfstoffe gegen Tuberkulose. Bundesgesundheitsblatt 2020, 63, 56–64. [Google Scholar] [CrossRef] [Green Version]
- De Bree, L.C.J.; Koeken, V.A.C.M.; Joosten, L.A.B.; Aaby, P.; Benn, C.S.; van Crevel, R.; Netea, M.G. Non-specific effects of vaccines: Current evidence and potential implications. Semin. Immunol. 2018, 39, 35–43. [Google Scholar] [CrossRef]
- Woo, M.; Wood, C.; Kwon, D.; Park, K.P.; Fejer, G.; Delorme, V. Mycobacterium tuberculosis Infection and Innate Responses in a New Model of Lung Alveolar Macrophages. Front. Immunol. 2018, 9, 438. [Google Scholar] [CrossRef]
- Parra, M.; Yang, A.L.; Lim, J.; Kolibab, K.; Derrick, S.; Cadieux, N.; Perera, L.P.; Jacobs, W.R.; Brennan, M.; Morris, S.L. Development of a Murine Mycobacterial Growth Inhibition Assay for Evaluating Vaccines against Mycobacterium tuberculosis. Clin. Vaccine Immunol. 2009, 16, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, Z.Y.; Huang, S.Y.; Petersen, E.; Song, H.Q.; Zhou, D.H.; Zhu, X.Q. Protective efficacy of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) adjuvated with recombinant IL-15 and IL-21 against experimental toxoplasmosis in mice. BMC Infect. Dis. 2014, 14, 487. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Bao, L.; Yang, X. Evaluation of immunogenicity and protective efficacy against Mycobacterium tuberculosis infection elicited by recombinant Mycobacterium bovis BCG expressing human Interleukin-12p70 and Early Secretory Antigen Target-6 fusion protein. Microbiol. Immunol. 2011, 55, 798–808. [Google Scholar] [CrossRef]
- Dowling, D.J.; Scott, E.A.; Scheid, A.; Bergelson, I.; Joshi, S.; Pietrasanta, C.; Brightman, S.; Sanchez-Schmitz, G.; Van Haren, S.D.; Ninković, J.; et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017, 140, 1339–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelzang, A.; Perdomo, C.; Zedler, U.; Kuhlmann, S.; Hurwitz, R.; Gengenbacher, M.; Kaufmann, S.H.E. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guérin ΔureC::hly vaccine’s superior protection against tuberculosis. J. Infect. Dis. 2014, 210, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Gröschel, M.I.; Sayes, F.; Shin, S.J.; Frigui, W.; Pawlik, A.; Orgeur, M.; Canetti, R.; Honoré, N.; Simeone, R.; van der Werf, T.S.; et al. Recombinant BCG Expressing ESX-1 of Mycobacterium marinum Combines Low Virulence with Cytosolic Immune Signaling and Improved TB Protection. Cell Rep. 2017, 18, 2752–2765. [Google Scholar] [CrossRef] [Green Version]
- De Martino, M.; Lodi, L.; Galli, L.; Chiappini, E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front. Pediatr. 2019, 7, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkert, S.; Schumann, R.R. RNA Sensing of Mycobacterium tuberculosis and Its Impact on TB Vaccination Strategies. Vaccines 2020, 8, 67. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines8010067
Burkert S, Schumann RR. RNA Sensing of Mycobacterium tuberculosis and Its Impact on TB Vaccination Strategies. Vaccines. 2020; 8(1):67. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines8010067
Chicago/Turabian StyleBurkert, Sanne, and Ralf R. Schumann. 2020. "RNA Sensing of Mycobacterium tuberculosis and Its Impact on TB Vaccination Strategies" Vaccines 8, no. 1: 67. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines8010067