2D Nanocomposite Membranes: Water Purification and Fouling Mitigation
Abstract
:1. Introduction
2. 2D Nanosheet Membranes
2.1. Nanostructured Materials in Membrane Technology
2.2. Nanosheet Materials
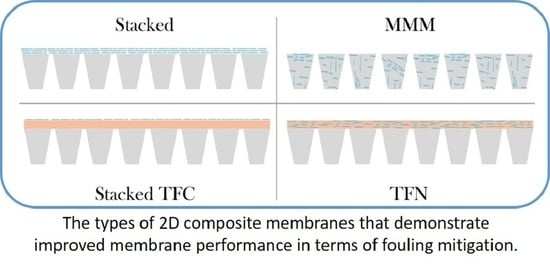
2.3. Types of Nanosheet Membranes
2.3.1. Stacked Nanosheet Membranes
2.3.2. Conventional Mixed Matrix Membranes
2.3.3. TFC and TFN Membranes
3. Antifouling Nanosheet Membranes
3.1. Fouling Mechanism
3.2. Antifouling Strategies
3.2.1. Hydrophilicity
3.2.2. Photocatalysis

4. Nanosheet Induced Fouling Mitigation
4.1. Non-Migratory Fouling Strategies
| Nanosheet | Type | Materials | Application | Foulant | WCA | Highlights | Ref. |
|---|---|---|---|---|---|---|---|
| GO | Stacked | PDDA, PAN(S) | NF | BSA, HA, SA | ∼50, increases with layers | LbL fabrication, FRR-HA = 91.2%, FRR-BSA = 92.7% | [197] |
| GO, rGO or MoS2 | Stacked | PES(S) | NF | BSA, SA | GO 40 ± 1.12 | MoS2 has highest flux and FRR | [196] |
| PMSA-GO | MMM | PVDF(S) | NF | BSA | zwitterions incorporated, better dispersion of GO, FRR = 95.3% | [160] | |
| WS2 | Stacked | AAO(S) | NF | BSA | FRR = 74.04% | [98] | |
| WS2 | MMM | CA(S) | UF | BSA | 63.3 ± 1.6 | FRR = 99.2 ± 0.8% | [198] |
| MoS2 | Stacked | PEI, PAA, PES(S) | FO | BSA | <90 | LbL fabrication | [85] |
| MoS2 or GO | MMM | PAI(S) | UF | HA, BSA | lower for MoS2 | higher FRR for MoS2 | [199] |
| MMT or LDH | TFN | PA(TF), PSf(S) | RO | BSA, DTAB, TA | MMT = , LDH = | different fouling behaviour because of nanosheet surface charge | [200] |
| gCN(H) | TFN | PDA(C), PA(TF), PES(S) | NF | BSA | >60 | FRR > 95% | [201] |
| gCN(H), rGO | Stacked | TiO2-NP, PVDF(S) | UF | BSA | 18 ± 8 | FRR = 86.1% | [202] |
| BN | MMM | PES(S) | NF | HA | 56 ± 2 | complete flux recovery | [29] |
| A-BN | Stacked TFC | PPA(TF), PES(S) | NF | SA, BSA | 25 ± 0.33 | Rir−SA = 2.1 ± 0.3% and Rir−BSA = 7.0 ± 2.0% | [203] |
| Ti3C2Tx | TFN | PA(TF), PSf(S) | RO | BSA | ∼70 | 11.1% flux decrease, resistance against chlorination | [30] |
| Ti3C2Tx | Stacked | AgNo3, PVDF(S) | NF | BSA, MB | 35 | FRR = 97% | [204] |
| Nanosheet | Type | Materials | Application | Organic Dye | Light | WCA | Highlights | Ref. |
|---|---|---|---|---|---|---|---|---|
| GO(CC) | Stacked | TiO2-NT(P), Ag-NP(CC), cellulose(S) | - | MB | Vis | - | complete flux decline, twice the flux of membrane without irradiation | [205] |
| GO(CC) | Stacked | TiO2-NP(P), MCE(S) | UF | DB, MO | UV, Vis | 11 | no irreversible fouling | [31] |
| N-GO(CC) | MMM | TiO2-NP(P), PSf(S) | UF | MB | UV, Vis | 59.2 ± 1.2 | FRR-UV = 94.6%, FRR-vis = 90.1% | [206] |
| rGO(CC), TiO2(P) | Stacked | Al2O3(S) | NF | MB, RhB, Congo Red, MO | Vis | 29.3 ± 3.4 | nearly constant permeance and selectivity | [207] |
| LDH(CC), gCN(H)(CC) | MMM | Ag3PO4(P), NH2−Ag3PO4(P), PES(S) | MF-MBR | BSA, AO7 | Vis | 40–50 | highest removal under light irradiation was for LDH-Ag | [208] |
| BP | MMM | PSf(S), SPEEK(S) | NF | MB | UV, Vis | increase | FRR = 85% | [209] |
4.2. Spreadable Fouling Strategies
4.3. Inorganic Fouling Strategies
5. Challenges
6. Summary and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| (C) | Cross-linker |
| (CC) | Co-Catalyst |
| (P) | Photocatalyst |
| (S) | Substrate |
| (TF) | Thin Film |
| AAO | Anodic aluminium oxide |
| AO7 | Acid orange 7 |
| AOP | Advanced oxidation processes |
| BN | Boron nitride |
| BP | Black phosphorus |
| BSA | Bovine Serum Albumin |
| CA | Cellulose Acetate |
| CB | Conduction band |
| CEC | Contaminants of concern |
| CNT | Carbon nanotube |
| COF | Covalent organic framework |
| DB | Diphenhydramine |
| DBP | Disinfection-by-products |
| DTAB | Dodecyltrimethylammonium bromide |
| FO | Forward osmosis |
| FRR | Flux recovery ratio |
| GA | Glutaraldehyde |
| GBN | Graphene-based-nanomaterials |
| gCN(H) | Graphitic carbon nitride |
| GO | Graphene oxide |
| HA | Humic acid |
| IP | Interfacial polymerization |
| LbL | Layer-by-Layer |
| LDH | Layered double hydroxide |
| MB | Methylene blue |
| MBR | Membrane Batch Reactor |
| MCE | Methyl Cellulose Ester |
| MF | Microfiltration |
| MMM | Mixed matrix membrane |
| MMT | Montmorillonite |
| MO | Methyl orange |
| MOF | Metal organic framework |
| MPD | M-phenylenediamine |
| MWCO | molecular weight cut-off |
| NF | Nanofiltration |
| NHE | Normal hydrogen electrode |
| NIPS | Non-solvent induced phase separation |
| NOM | Natural organic matter |
| NP | Nanoparticle |
| NT | Nanotube |
| NW | Nanowire |
| OCA | Oil contact angle |
| PA | Polyamide |
| PAA | Polyacrylic acid |
| PAI | Polyamide-imide |
| PAN | Polyacrylonitrile |
| PDA | Polydopamine |
| PDDA | Poly(diallyldimethylammonium chloride) |
| PEI | Polyethylenimine |
| PEN | Poly(arylene ether nitrile) |
| PES | Polyethersulfone |
| PMR | Photocatalytic membrane reactor |
| PMSA | Poly 2-(methacryloyloxy)ethyl dimethyl-(3-sulfo-propyl)ammonium hydroxide |
| PPA | Polypiperazine amide |
| PSf | Polysulfone |
| PVA | Polyvinyl alcohol |
| PVDF | Polyvinylidene difluoride |
| PVDF-HFP | Poly(vinylidene fluoride-co-hexafluoropropylene) |
| rGO | Reduced graphene oxide |
| RhB | Rhodamine B |
| RO | Reverse osmosis |
| ROS | Reactive oxygen species |
| SA | Sodium alginate |
| SDG | Sustainable Development Goal |
| SDS | Sodium dodecyl sulfate |
| SPEEK | Sulfonated poly(ether ether ketone) |
| TA | Tannic acid |
| TEOA | Triethanolamine |
| TFC | Thin film composite |
| TFN | Thin film nanocomposite |
| TMC | trimesoyl chloride |
| TMD | Transition metal dichalcegonides |
| TMO | Tranisition metal oxides |
| UF | Ultrafiltration |
| VB | Valence band |
| WCA | Water contact angle |
References
- World Health Organization; United Nations Children’s Fund. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; World Health Organization; The United Nations Children’s Fund: Geneva, Switzerland, 2017; p. 3. [Google Scholar]
- UNESCO. UN-Water, 2020: United Nations World Water Development Report 2020: Water and Climate Change; UNESCO: Paris, France, 2020; p. iv-2. [Google Scholar]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging pollutants in the urban water cycle in Latin America: A review of the current literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Im, J.K.; Hwang, M.Y.; Lee, E.H.; Noh, H.R.; Yu, S.J. Pharmaceutical compounds in tributaries of the Han River watershed, South Korea. Environ. Res. 2020, 188, 109758. [Google Scholar] [CrossRef] [PubMed]
- Čelić, M.; Gros, M.; Farré, M.; Barceló, D.; Petrović, M. Pharmaceuticals as chemical markers of wastewater contamination in the vulnerable area of the Ebro Delta (Spain). Sci. Total Environ. 2019, 652, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Houtman, C.J. Emerging contaminants in surface waters and their relevance for the production of drinking water in Europe. J. Integr. Environ. Sci. 2010, 7, 271–295. [Google Scholar] [CrossRef]
- Akhondi, E.; Zamani, F.; Tng, K.; Leslie, G.; Krantz, W.; Fane, A.; Chew, J. The Performance and Fouling Control of Submerged Hollow Fiber (HF) Systems: A Review. Appl. Sci. 2017, 7, 765. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2016, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Li, B.; Zhang, T.; Li, X.Y. Performance of nanofiltration membrane in rejecting trace organic compounds: Experiment and model prediction. Desalination 2015, 370, 7–16. [Google Scholar] [CrossRef]
- Wang, J.; Cahyadi, A.; Wu, B.; Pee, W.; Fane, A.G.; Chew, J.W. The roles of particles in enhancing membrane filtration: A review. J. Membr. Sci. 2020, 595, 117570. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Charfi, A.; Lesage, G.; Heran, M.; Kim, J. Membrane bioreactors for wastewater treatment: A review of mechanical cleaning by scouring agents to control membrane fouling. Chem. Eng. J. 2017, 307, 897–913. [Google Scholar] [CrossRef]
- Petrus, H.; Li, H.; Chen, V.; Norazman, N. Enzymatic cleaning of ultrafiltration membranes fouled by protein mixture solutions. J. Membr. Sci. 2008, 325, 783–792. [Google Scholar] [CrossRef]
- Huotari, H.; Trägårdh, G.; Huisman, I. Crossflow Membrane Filtration Enhanced by an External DC Electric Field: A Review. Chem. Eng. Res. Des. 1999, 77, 461–468. [Google Scholar] [CrossRef]
- Zularisam, A.; Ismail, A.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Hou, L.; Tang, B.; Wu, P. A self-protected self-cleaning ultrafiltration membrane by using polydopamine as a free-radical scavenger. J. Membr. Sci. 2015, 490, 120–128. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and Membrane Hydrophilic Modification to Reduce Membrane Fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef]
- Nishimoto, S.; Takiguchi, T.; Kameshima, Y.; Miyake, M. Underwater superoleophobicity of Nb2O5 photocatalyst surface. Chem. Phys. Lett. 2019, 726, 34–38. [Google Scholar] [CrossRef]
- Ying, Y.; Ying, W.; Li, Q.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Recent advances of nanomaterial-based membrane for water purification. Appl. Mater. Today 2017, 7, 144–158. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Xue, J.; Hou, J.; Ding, L.; Wang, H. Enhanced water flux through graphitic carbon nitride nanosheets membrane by incorporating polyacrylic acid. AIChE J. 2018, 64, 2181–2188. [Google Scholar] [CrossRef]
- Jo, Y.K.; Lee, J.M.; Son, S.; Hwang, S.J. 2D inorganic nanosheet-based hybrid photocatalysts: Design, applications, and perspectives. J. Photochem. Photobiol. C 2019, 40, 150–190. [Google Scholar] [CrossRef]
- Kim, S.; Wang, H.; Lee, Y.M. 2D Nanosheets and Their Composite Membranes for Water, Gas, and Ion Separation. Angew. Chem. Int. Ed. 2019, 58, 17512–17527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.Y.L.; Tai, M.H.; Juay, J.; Liu, Z.; Sun, D. A study on the performance of self-cleaning oil–water separation membrane formed by various TiO2 nanostructures. Sep. Purif. Technol. 2015, 156, 942–951. [Google Scholar] [CrossRef]
- Low, Z.X.; Ji, J.; Blumenstock, D.; Chew, Y.M.; Wolverson, D.; Mattia, D. Fouling resistant 2D boron nitride nanosheet—PES nanofiltration membranes. J. Membr. Sci. 2018, 563, 949–956. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Zhang, J.; Huang, H.; Wu, S.; Yang, Y. Novel thin-film reverse osmosis membrane with MXene Ti3C2T embedded in polyamide to enhance the water flux, anti-fouling and chlorine resistance for water desalination. J. Membr. Sci. 2020, 603, 118036. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M. Graphene oxide based ultrafiltration membranes for photocatalytic degradation of organic pollutants in salty water. Water Res. 2015, 77, 179–190. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, G.; Liu, Y.; Zhang, X. Graphene-based antimicrobial nanomaterials: Rational design and applications for water disinfection and microbial control. Environ. Sci. Nano 2017, 4, 2248–2266. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Hou, J.; Zhang, Y.; Liu, J.; der Bruggen, B.V. Graphene-based antimicrobial polymeric membranes: A review. J. Mater. Chem. A 2017, 5, 6776–6793. [Google Scholar] [CrossRef]
- Pokropivny, V.; Skorokhod, V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Ying, Y.; Yang, Y.; Ying, W.; Peng, X. Two-dimensional materials for novel liquid separation membranes. Nanotechnology 2016, 27, 332001. [Google Scholar] [CrossRef]
- Qin, A.; Li, X.; Zhao, X.; Liu, D.; He, C. Engineering a Highly Hydrophilic PVDF Membrane via Binding TiO2 Nanoparticles and a PVA Layer onto a Membrane Surface. ACS Appl. Mater. Interfaces 2015, 7, 8427–8436. [Google Scholar] [CrossRef]
- Majumder, M.; Chopra, N.; Hinds, B.J. Mass Transport through Carbon Nanotube Membranes in Three Different Regimes: Ionic Diffusion and Gas and Liquid Flow. ACS Nano 2011, 5, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; de Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Correction to Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 12, 526. [Google Scholar] [CrossRef]
- Tao, Q.; He, H.; Frost, R.L.; Yuan, P.; Zhu, J. Nanomaterials based upon silylated layered double hydroxides. Appl. Surf. Sci. 2009, 255, 4334–4340. [Google Scholar] [CrossRef]
- Chatterjee, S.; Luo, Z.; Acerce, M.; Yates, D.M.; Johnson, A.T.C.; Sneddon, L.G. Chemical Vapor Deposition of Boron Nitride Nanosheets on Metallic Substrates via Decaborane/Ammonia Reactions. Chem. Mater. 2011, 23, 4414–4416. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the Local Chemical Structure of Graphene Oxide and Reduced Graphene Oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Stylianakis, M.; Viskadouros, G.; Polyzoidis, C.; Veisakis, G.; Kenanakis, G.; Kornilios, N.; Petridis, K.; Kymakis, E. Updating the Role of Reduced Graphene Oxide Ink on Field Emission Devices in Synergy with Charge Transfer Materials. Nanomaterials 2019, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Kreissl, H.T.; Li, M.M.J.; Peng, Y.K.; Nakagawa, K.; Hooper, T.J.N.; Hanna, J.V.; Shepherd, A.; Wu, T.S.; Soo, Y.L.; Tsang, S.C.E. Structural Studies of Bulk to Nanosize Niobium Oxides with Correlation to Their Acidity. J. Am. Chem. Soc. 2017, 139, 12670–12680. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2013, 26, 992–1005. [Google Scholar] [CrossRef]
- Arab, A.; Li, Q. Anisotropic thermoelectric behavior in armchair and zigzag mono- and fewlayer MoS2 in thermoelectric generator applications. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohwada, M.; Kimoto, K.; Mizoguchi, T.; Ebina, Y.; Sasaki, T. Atomic structure of titania nanosheet with vacancies. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-zadeh, K.; Ou, J.Z.; Daeneke, T.; Mitchell, A.; Sasaki, T.; Fuhrer, M.S. Two dimensional and layered transition metal oxides. Appl. Mater. Today 2016, 5, 73–89. [Google Scholar] [CrossRef]
- Takagaki, A.; Tagusagawa, C.; Hayashi, S.; Hara, M.; Domen, K. Nanosheets as highly active solid acid catalysts for green chemical syntheses. Energy Environ. Sci. 2010, 3, 82–93. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Karahan, H.E.; Goh, K.; Zhang, C.J.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y.; et al. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 1906697. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Wang, Z.; von dem Bussche, A.; Qiu, Y.; Valentin, T.M.; Gion, K.; Kane, A.B.; Hurt, R.H. Chemical Dissolution Pathways of MoS2 Nanosheets in Biological and Environmental Media. Environ. Sci. Technol. 2016, 50, 7208–7217. [Google Scholar] [CrossRef] [Green Version]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Algara-Siller, G.; Severin, N.; Chong, S.Y.; Björkman, T.; Palgrave, R.G.; Laybourn, A.; Antonietti, M.; Khimyak, Y.Z.; Krasheninnikov, A.V.; Rabe, J.P.; et al. Triazine-Based Graphitic Carbon Nitride: A Two-Dimensional Semiconductor. Angew. Chem. Int. Ed. 2014, 53, 7450–7455. [Google Scholar] [CrossRef] [PubMed]
- Kouvetakis, J.; Todd, M.; Wilkens, B.; Bandari, A.; Cave, N. Novel Synthetic Routes to Carbon-Nitrogen Thin Films. Chem. Mater. 1994, 6, 811–814. [Google Scholar] [CrossRef]
- He, G.; Dong, T.; Yang, Z.; Ohlckers, P. Tuning 2D Black Phosphorus: Defect Tailoring and Surface Functionalization. Chem. Mater. 2019, 31, 9917–9938. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Jiang, D.; Duan, H.; Sun, Z.; Zhang, M.; Jin, H.; Guan, R.; Liu, Y.; Chen, M.; et al. Stabilizing black phosphorus nanosheets via edge-selective bonding of sacrificial C60 molecules. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [Green Version]
- Zirehpour, A.; Rahimpour, A.; Khoshhal, S.; Firouzjaei, M.D.; Ghoreyshi, A.A. The impact of MOF feasibility to improve the desalination performance and antifouling properties of FO membranes. RSC Adv. 2016, 6, 70174–70185. [Google Scholar] [CrossRef]
- Ashworth, D.J.; Foster, J.A. Metal–organic framework nanosheets (MONs): A new dimension in materials chemistry. J. Mater. Chem. A 2018, 6, 16292–16307. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Yang, W. 2D Metal-Organic Framework Materials for Membrane-Based Separation. Adv. Mater. Interfaces 2019, 7, 1901514. [Google Scholar] [CrossRef]
- Lu, P.; Liu, Y.; Zhou, T.; Wang, Q.; Li, Y. Recent advances in layered double hydroxides (LDHs) as two-dimensional membrane materials for gas and liquid separations. J. Membr. Sci. 2018, 567, 89–103. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Corso, M. Boron Nitride Nanomesh. Science 2004, 303, 217–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.Q.; Wu, L.; Zhu, Y.; Watanabe, K.; Taniguchi, T. Structure of chemically derived mono- and few-atomic-layer boron nitride sheets. Appl. Phys. Lett. 2008, 93, 223103. [Google Scholar] [CrossRef]
- Wang, L.; Boutilier, M.S.H.; Kidambi, P.R.; Jang, D.; Hadjiconstantinou, N.G.; Karnik, R. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. 2017, 12, 509–522. [Google Scholar] [CrossRef]
- Kim, H.J.; Lim, M.Y.; Jung, K.H.; Kim, D.G.; Lee, J.C. High-performance reverse osmosis nanocomposite membranes containing the mixture of carbon nanotubes and graphene oxides. J. Mater. Chem. A 2015, 3, 6798–6809. [Google Scholar] [CrossRef]
- Ali, F.A.A.; Alam, J.; Shukla, A.K.; Alhoshan, M.; Khaled, J.M.; Al-Masry, W.A.; Alharbi, N.S.; Alam, M. Graphene oxide-silver nanosheet-incorporated polyamide thin-film composite membranes for antifouling and antibacterial action against Escherichia coli and bovine serum albumin. J. Ind. Eng. Chem. 2019, 80, 227–238. [Google Scholar] [CrossRef]
- Lim, S.; Park, K.H.; Tran, V.H.; Akther, N.; Phuntsho, S.; Choi, J.Y.; Shon, H.K. Size-controlled graphene oxide for highly permeable and fouling-resistant outer-selective hollow fiber thin-film composite membranes for forward osmosis. J. Membr. Sci. 2020, 609, 118171. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.O. Electric fields line up graphene oxide. Nat. Mater. 2014, 13, 325–326. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, H.W.; Yoon, S.M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Morelos-Gomez, A.; Cruz-Silva, R.; Muramatsu, H.; Ortiz-Medina, J.; Araki, T.; Fukuyo, T.; Tejima, S.; Takeuchi, K.; Hayashi, T.; Terrones, M.; et al. Effective NaCl and dye rejection of hybrid graphene oxide/graphene layered membranes. Nat. Nanotechnol. 2017, 12, 1083–1088. [Google Scholar] [CrossRef]
- Rao, G.; Zhang, Q.; Zhao, H.; Chen, J.; Li, Y. Novel titanium dioxide/iron (III) oxide/graphene oxide photocatalytic membrane for enhanced humic acid removal from water. Chem. Eng. J. 2016, 302, 633–640. [Google Scholar] [CrossRef]
- Gao, B.; Liu, L.; Liu, J.; Yang, F. Photocatalytic degradation of 2,4,6-tribromophenol over Fe-doped ZnIn2S4: Stable activity and enhanced debromination. Appl. Catal. B 2013, 129, 89–97. [Google Scholar] [CrossRef]
- Li, M.N.; Sun, X.F.; Wang, L.; Wang, S.Y.; Afzal, M.Z.; Song, C.; Wang, S.G. Forward osmosis membranes modified with laminar MoS2 nanosheet to improve desalination performance and antifouling properties. Desalination 2018, 436, 107–113. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Fan, X.; Zhang, H.; Yu, H.; Quan, X. A multifunctional graphene-based nanofiltration membrane under photo-assistance for enhanced water treatment based on layer-by-layer sieving. Appl. Catal. B 2018, 224, 204–213. [Google Scholar] [CrossRef]
- Peeters, J.; Boom, J.; Mulder, M.; Strathmann, H. Retention measurements of nanofiltration membranes with electrolyte solutions. J. Membr. Sci. 1998, 145, 199–209. [Google Scholar] [CrossRef]
- Mi, B. Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Liang, B.; Liu, Y.; Xu, T.; Wang, L.; Cao, B.; Pan, K. Graphene Oxide as an Effective Barrier on a Porous Nanofibrous Membrane for Water Treatment. ACS Appl. Mater. Interfaces 2016, 8, 6211–6218. [Google Scholar] [CrossRef]
- Yeh, C.N.; Raidongia, K.; Shao, J.; Yang, Q.H.; Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2015, 7, 166–170. [Google Scholar] [CrossRef]
- Nakagawa, K.; Araya, S.; Kunimatsu, M.; Yoshioka, T.; Shintani, T.; Kamio, E.; Matsuyama, H. Fabrication of Stacked Graphene Oxide Nanosheet Membranes Using Triethanolamine as a Crosslinker and Mild Reducing Agent for Water Treatment. Membranes 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir-Blodgett Assembly of Graphite Oxide Single Layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.; Jiang, W.; Karahan, H.E.; Zhai, S.; Wei, L.; Yu, D.; Fane, A.G.; Wang, R.; Chen, Y. All-Carbon Nanoarchitectures as High-Performance Separation Membranes with Superior Stability. Adv. Funct. Mater. 2015, 25, 7348–7359. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, X.; Yang, W.; Wang, Y.; Simon, G.P.; Li, D. Controllable corrugation of chemically converted graphene sheets in water and potential application for nanofiltration. Chem. Commun. 2011, 47, 5810. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, M.; Ding, H.; Fu, K.; Fan, Y. A reduced graphene oxide nanofiltration membrane intercalated by well-dispersed carbon nanotubes for drinking water purification. Nanoscale 2016, 8, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ying, Y.; Huang, H.; Song, Z.; Mao, Y.; Xu, Z.; Peng, X. Ultrafast Molecule Separation through Layered WS2 Nanosheet Membranes. ACS Nano 2014, 8, 6304–6311. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Chen, Y.; Yan, X.; Wang, Y.; Lang, W.Z. Highly Stable and Antibacterial Two-Dimensional Tungsten Disulfide Lamellar Membrane for Water Filtration. ChemSusChem 2018, 12, 275–282. [Google Scholar] [CrossRef]
- Sun, L.; Huang, H.; Peng, X. Laminar MoS2 membranes for molecule separation. Chem. Commun. 2013, 49, 10718. [Google Scholar] [CrossRef] [PubMed]
- Hirunpinyopas, W.; Prestat, E.; Worrall, S.D.; Haigh, S.J.; Dryfe, R.A.W.; Bissett, M.A. Desalination and Nanofiltration through Functionalized Laminar MoS2 Membranes. ACS Nano 2017, 11, 11082–11090. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Q.; Zheng, S.; Urban, J.J.; Li, S.; Mi, B. Understanding the Aqueous Stability and Filtration Capability of MoS2 Membranes. Nano Lett. 2017, 17, 7289–7298. [Google Scholar] [CrossRef]
- Jiang, J.W.; Qi, Z.; Park, H.S.; Rabczuk, T. Elastic bending modulus of single-layer molybdenum disulfide (MoS2): Finite thickness effect. Nanotechnology 2013, 24, 435705. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Wei, Y.; Deng, J.; Ding, L.; Li, Z.K.; Wang, H. Self-Crosslinked MXene (Ti3C2Tx) Membranes with Good Antiswelling Property for Monovalent Metal Ion Exclusion. ACS Nano 2019, 13, 10535–10544. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, L.; Liu, Y.; Wu, Y.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 2020, 3, 296–302. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, V.; Farimani, A.B. Water Desalination with Two-Dimensional Metal–Organic Framework Membranes. Nano Lett. 2019, 19, 8638–8643. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sera, T.; Kunimatsu, M.; Yamashita, H.; Yoshioka, T.; Shintani, T.; Kamio, E.; Tsang, S.C.E.; Matsuyama, H. Two-dimensional niobate nanosheet membranes for water treatment: Effect of nanosheet preparation method on membrane performance. Sep. Purif. Technol. 2019, 219, 222–229. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Nakagawa, K.; Peng, Y.K.; Koito, Y.; Zheng, J.; Tsang, S.C.E. Niobium oxides: Correlation of acidity with structure and catalytic performance in sucrose conversion to 5-hydroxymethylfurfural. J. Catal. 2016, 338, 329–339. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yamashita, H.; Saeki, D.; Yoshioka, T.; Shintani, T.; Kamio, E.; Kreissl, H.T.; Tsang, S.C.E.; Sugiyama, S.; Matsuyama, H. Niobate nanosheet membranes with enhanced stability for nanofiltration. Chem. Commun. 2017, 53, 7929–7932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunimatsu, M.; Nakagawa, K.; Yoshioka, T.; Shintani, T.; Yasui, T.; Kamio, E.; Tsang, S.C.E.; Li, J.; Matsuyama, H. Design of niobate nanosheet-graphene oxide composite nanofiltration membranes with improved permeability. J. Membr. Sci. 2020, 595, 117598. [Google Scholar] [CrossRef]
- Hu, M.; Cui, Z.; Li, J.; Zhang, L.; Mo, Y.; Dlamini, D.S.; Wang, H.; He, B.; Li, J.; Matsuyama, H. Ultra-low graphene oxide loading for water permeability, antifouling and antibacterial improvement of polyethersulfone/sulfonated polysulfone ultrafiltration membranes. J. Colloid Interface Sci. 2019, 552, 319–331. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, S.; Chen, G.; Xiao, K.; Li, M.; Gao, Y.; Liang, S.; Huang, X. Superhydrophilic and oleophobic membrane functionalized with heterogeneously tailored two-dimensional layered double hydroxide nanosheets for antifouling. J. Membr. Sci. 2019, 577, 165–175. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Sriyamunadevi, T.; Raajenthiren, M. Effect of silica particles on cellulose acetate blend ultrafiltration membranes: Part I. Sep. Purif. Technol. 2008, 64, 38–47. [Google Scholar] [CrossRef]
- Liu, F.; Abed, M.M.; Li, K. Preparation and characterization of poly(vinylidene fluoride) (PVDF) based ultrafiltration membranes using nano γ-Al2O3. J. Membr. Sci. 2011, 366, 97–103. [Google Scholar] [CrossRef]
- Choi, J.H.; Jegal, J.; Kim, W.N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Zhou, Z.; Zhang, L.; Chen, H. Improving the antifouling property of polysulfone ultrafiltration membrane by incorporation of isocyanate-treated graphene oxide. Phys. Chem. Chem. Phys. 2013, 15, 9084. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Shan, M.; Li, Y.; Li, B.; Niu, J.; Zhou, B.; Qian, X. Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2014, 458, 1–13. [Google Scholar] [CrossRef]
- Ma, M.Q.; Zhang, C.; Zhu, C.Y.; Huang, S.; Yang, J.; Xu, Z.K. Nanocomposite membranes embedded with functionalized MoS2 nanosheets for enhanced interfacial compatibility and nanofiltration performance. J. Membr. Sci. 2019, 591, 117316. [Google Scholar] [CrossRef]
- Kaneda, M.; Lu, X.; Cheng, W.; Zhou, X.; Bernstein, R.; Zhang, W.; Kimura, K.; Elimelech, M. Photografting Graphene Oxide to Inert Membrane Materials to Impart Antibacterial Activity. Environ. Sci. Technol. Lett. 2019, 6, 141–147. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, T.; Shi, J.; Wang, W.; Teng, K.; Qian, X.; Shan, M.; Deng, H.; Tian, X.; Li, C.; et al. Manipulating Migration Behavior of Magnetic Graphene Oxide via Magnetic Field Induced Casting and Phase Separation toward High-Performance Hybrid Ultrafiltration Membranes. ACS Appl. Mater. Interfaces 2016, 8, 18418–18429. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, C.; Huang, Q.; Liu, H.; Hao, J.; Song, L. Magnetic field induced orderly arrangement of Fe3O4/GO composite particles for preparation of Fe3O4/GO/PVDF membrane. J. Membr. Sci. 2018, 548, 184–193. [Google Scholar] [CrossRef]
- Roh, I.J.; Greenberg, A.R.; Khare, V.P. Synthesis and characterization of interfacially polymerized polyamide thin films. Desalination 2006, 191, 279–290. [Google Scholar] [CrossRef]
- Kang, G.D.; Gao, C.J.; Chen, W.D.; Jie, X.M.; Cao, Y.M.; Yuan, Q. Study on hypochlorite degradation of aromatic polyamide reverse osmosis membrane. J. Membr. Sci. 2007, 300, 165–171. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Motsa, M.M.; Mamba, B.B.; Verliefde, A.R. Combined colloidal and organic fouling of FO membranes: The influence of foulant–foulant interactions and ionic strength. J. Membr. Sci. 2015, 493, 539–548. [Google Scholar] [CrossRef]
- Motsa, M.M.; Mamba, B.B.; D’Haese, A.; Hoek, E.M.; Verliefde, A.R. Organic fouling in forward osmosis membranes: The role of feed solution chemistry and membrane structural properties. J. Membr. Sci. 2014, 460, 99–109. [Google Scholar] [CrossRef]
- Kimura, K.; Iwase, T.; Kita, S.; Watanabe, Y. Influence of residual organic macromolecules produced in biological wastewater treatment processes on removal of pharmaceuticals by NF/RO membranes. Water Res. 2009, 43, 3751–3758. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, B.; Zhu, T.; Shi, J.; Xu, Z.; Hu, C.; Wang, J. Facile and low-cost approach towards a PVDF ultrafiltration membrane with enhanced hydrophilicity and antifouling performance via graphene oxide/water-bath coagulation. RSC Adv. 2015, 5, 7880–7889. [Google Scholar] [CrossRef]
- Qin, D.; Liu, Z.; Bai, H.; Sun, D.D.; Song, X. A new nano-engineered hierarchical membrane for concurrent removal of surfactant and oil from oil-in-water nanoemulsion. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Kwan, S.E.; Bar-Zeev, E.; Elimelech, M. Biofouling in forward osmosis and reverse osmosis: Measurements and mechanisms. J. Membr. Sci. 2015, 493, 703–708. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Navale, G.R.; Rout, C.S.; Gohil, K.N.; Dharne, M.S.; Late, D.J.; Shinde, S.S. Oxidative and membrane stress-mediated antibacterial activity of WS2and rGO-WS2nanosheets. RSC Adv. 2015, 5, 74726–74733. [Google Scholar] [CrossRef]
- Kim, T.I.; Kwon, B.; Yoon, J.; Park, I.J.; Bang, G.S.; Park, Y.; Seo, Y.S.; Choi, S.Y. Antibacterial Activities of Graphene Oxide–Molybdenum Disulfide Nanocomposite Films. ACS Appl. Mater. Interfaces 2017, 9, 7908–7917. [Google Scholar] [CrossRef]
- Lu, X.; Feng, X.; Werber, J.R.; Chu, C.; Zucker, I.; Kim, J.H.; Osuji, C.O.; Elimelech, M. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc. Natl. Acad. Sci. USA 2017, 114, E9793–E9801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, T.; Wallace, A.F.; Zhao, S.; Wang, Z. Mineral scaling in membrane desalination: Mechanisms, mitigation strategies, and feasibility of scaling-resistant membranes. J. Membr. Sci. 2019, 579, 52–69. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard V, J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B 2015, 176–177, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Law, K.Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, T.; Crawshaw, J.; Liu, T.; Graham, N. Ultrafiltration and nanofiltration membrane fouling by natural organic matter: Mechanisms and mitigation by pre-ozonation and pH. Water Res. 2018, 139, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Bui, N.N.; Meyering, M.T.; Hamlin, T.J.; McCutcheon, J.R. Novel hydrophilic nylon 6,6 microfiltration membrane supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2013, 437, 141–149. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface Modification of Water Purification Membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the Surface Roughness on Sliding Angles of Water Droplets on Superhydrophobic Surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, S.; Li, F.; Zhao, X.; Wang, W. Coexistence of superhydrophilicity and superoleophobicity: Theory, experiments and applications in oil/water separation. J. Mater. Chem. A 2018, 6, 15057–15063. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Sawai, Y.; Nishimoto, S.; Kameshima, Y.; Fujii, E.; Miyake, M. Photoinduced Underwater Superoleophobicity of TiO2 Thin Films. Langmuir 2013, 29, 6784–6789. [Google Scholar] [CrossRef]
- Sun, T.; Wang, G.; Feng, L.; Liu, B.; Ma, Y.; Jiang, L.; Zhu, D. Reversible Switching between Superhydrophilicity and Superhydrophobicity. Angew. Chem. Int. Ed. 2004, 43, 357–360. [Google Scholar] [CrossRef]
- Krupenkin, T.N.; Taylor, J.A.; Schneider, T.M.; Yang, S. From Rolling Ball to Complete Wetting: The Dynamic Tuning of Liquids on Nanostructured Surfaces. Langmuir 2004, 20, 3824–3827. [Google Scholar] [CrossRef]
- Zhu, X.; Tu, W.; Wee, K.H.; Bai, R. Effective and low fouling oil/water separation by a novel hollow fiber membrane with both hydrophilic and oleophobic surface properties. J. Membr. Sci. 2014, 466, 36–44. [Google Scholar] [CrossRef]
- Zhu, X.; Loo, H.E.; Bai, R. A novel membrane showing both hydrophilic and oleophobic surface properties and its non-fouling performances for potential water treatment applications. J. Membr. Sci. 2013, 436, 47–56. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Guan, J.; Cao, J.; Zhang, R.; He, M.; Gao, K.; Zhou, L.; Jiang, Z. 2D Heterostructure Membranes with Sunlight-Driven Self-Cleaning Ability for Highly Efficient Oil-Water Separation. Adv. Funct. Mater. 2018, 28, 1706545. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; der Bruggen, B.V. Superhydrophilic and underwater superoleophobic membranes—A review of synthesis methods. Prog. Polym. Sci. 2019, 98, 101166. [Google Scholar] [CrossRef]
- Kong, S.; young Lim, M.; Shin, H.; Baik, J.H.; Lee, J.C. High-flux and antifouling polyethersulfone nanocomposite membranes incorporated with zwitterion-functionalized graphene oxide for ultrafiltration applications. J. Ind. Eng. Chem. 2020, 84, 131–140. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, H.; Rahimi, A. Zwitterion functionalized graphene oxide/polyamide thin film nanocomposite membrane: Towards improved anti-fouling performance for reverse osmosis. Desalination 2018, 433, 94–107. [Google Scholar] [CrossRef]
- Rahimi, A.; Mahdavi, H. Zwitterionic-functionalized GO/PVDF nanocomposite membranes with improved anti-fouling properties. J. Water Process Eng. 2019, 32, 100960. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and Photoinduced Hydrophilicity of Various Metal Oxide Thin Films. Chem. Mater. 2002, 14, 2812–2816. [Google Scholar] [CrossRef]
- Shibata, T.; Takanashi, G.; Nakamura, T.; Fukuda, K.; Ebina, Y.; Sasaki, T. Titanoniobate and niobate nanosheet photocatalysts: Superior photoinduced hydrophilicity and enhanced thermal stability of unilamellar Nb3O8nanosheet. Energy Environ. Sci. 2011, 4, 535–542. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yamaguchi, K.; Yamada, K.; Sotowa, K.I.; Sugiyama, S.; Adachi, M. Synthesis and Characterization of Surface-Functionalized Layered Titanate Nanosheets Using Lamellar Self-Assembly as a Template. Eur. J. Inorg. Chem. 2012, 2012, 2741–2748. [Google Scholar] [CrossRef]
- Karunakaran, C.; Vinayagamoorthy, P.; Jayabharathi, J. Nonquenching of Charge Carriers by Fe3O4 Core in Fe3O4/ZnO Nanosheet Photocatalyst. Langmuir 2014, 30, 15031–15039. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Wu, L.; Duan, Z.; Jiang, J.; Zhao, L. Synthesis of a Thin-Layer MnO2 Nanosheet-Coated Fe3O4 Nanocomposite as a Magnetically Separable Photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef]
- Nakagawa, K.; Jia, T.; Zheng, W.; Fairclough, S.M.; Katoh, M.; Sugiyama, S.; Tsang, S.C.E. Enhanced photocatalytic hydrogen evolution from water by niobate single molecular sheets and ensembles. Chem. Commun. 2014, 50, 13702–13705. [Google Scholar] [CrossRef]
- Lei, R.; Ni, H.; Chen, R.; Zhang, B.; Zhan, W.; Li, Y. Hydrothermal synthesis of WO3/Fe2O3 nanosheet arrays on iron foil for photocatalytic degradation of methylene blue. J. Mater. Sci. Mater. Electron. 2017, 28, 10481–10487. [Google Scholar] [CrossRef]
- Jia, T.; Li, M.M.J.; Ye, L.; Wiseman, S.; Liu, G.; Qu, J.; Nakagawa, K.; Tsang, S.C.E. The remarkable activity and stability of a dye-sensitized single molecular layer MoS2ensemble for photocatalytic hydrogen production. Chem. Commun. 2015, 51, 13496–13499. [Google Scholar] [CrossRef]
- Lin, B.; Sun, P.; Zhou, Y.; Jiang, S.; Gao, B.; Chen, Y. Interstratified nanohybrid assembled by alternating cationic layered double hydroxide nanosheets and anionic layered titanate nanosheets with superior photocatalytic activity. J. Hazard. Mater. 2014, 280, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Eren, T.; Atar, N.; Yola, M.L.; Parlak, C.; Karimi-Maleh, H. CoFe2O4@TiO2 decorated reduced graphene oxide nanocomposite for photocatalytic degradation of chlorpyrifos. J. Mol. Liq. 2015, 208, 122–129. [Google Scholar] [CrossRef]
- Ye, T.; Chen, W.; Xu, H.; Geng, N.; Cai, Y. Preparation of TiO2/graphene composite with appropriate N-doping ratio for humic acid removal. J. Mater. Sci. 2017, 53, 613–625. [Google Scholar] [CrossRef]
- Khadgi, N.; Upreti, A.R.; Li, Y. Simultaneous bacterial inactivation and degradation of an emerging pollutant under visible light by ZnFe2O4 co-modified with Ag and rGO. RSC Adv. 2017, 7, 27007–27016. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Cui, L.; Liu, C.; Zhang, F.; Li, K.; Xia, L.; Su, X.; Feng, J.; Zhu, Y.; Sun, J. Fabrication of 3D ultra-light graphene aerogel/Bi2WO6 composite with excellent photocatalytic performance: A promising photocatalysts for water purification. J. Taiwan Inst. Chem. Eng. 2019, 97, 288–296. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, M.W.; Xie, W.J.; Sun, L.; Chen, Y.; Lei, W.W. Efficient photocatalytic reduction of aqueous Cr(vi) over porous BNNSs/TiO2 nanocomposites under visible light irradiation. Catal. Sci. Technol. 2016, 6, 8309–8313. [Google Scholar] [CrossRef]
- Yu, S.; Wang, J.; Song, S.; Sun, K.; Li, J.; Wang, X.; Chen, Z.; Wang, X. One-pot synthesis of graphene oxide and Ni-Al layered double hydroxides nanocomposites for the efficient removal of U(VI) from wastewater. Sci. China Chem. 2017, 60, 415–422. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Peña-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. NPJ Clean Water 2020, 3. [Google Scholar] [CrossRef] [Green Version]
- Kokkinos, P.; Mantzavinos, D.; Venieri, D. Current Trends in the Application of Nanomaterials for the Removal of Emerging Micropollutants and Pathogens from Water. Molecules 2020, 25, 2016. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.S.; Chen, C.H.; Chen, Y.R.; Huang, P.H.; Tung, K.L. Phosphorus-doped g-C3N4 integrated photocatalytic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 580, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.K.; da Costa, J.C.D. Recent progresses on fabrication of photocatalytic membranes for water treatment. Catal. Today 2014, 230, 47–54. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Luo, B.; Liu, G.; Wang, L. Recent advances in 2D materials for photocatalysis. Nanoscale 2016, 8, 6904–6920. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Eftekhari, E.; Huo, Z.; Li, X.; Tade, M.O.; Yan, C.; Yan, Z.; Li, C.; Li, Q.; et al. High performance heterojunction photocatalytic membranes formed by embedding Cu2O and TiO2 nanowires in reduced graphene oxide. Catal. Sci. Technol. 2018, 8, 1704–1711. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhu, Y.; Long, J.; Ding, Z.; Yuan, R.; Li, Z.; Xu, C. In situ construction of layered graphene-based nanofiltration membranes with interlayer photocatalytic purification function and their application for water treatment. Environ. Sci. Nano 2019, 6, 2195–2202. [Google Scholar] [CrossRef]
- Li, T.; Gao, Y.; Zhou, J.; Zhang, M.; Fu, X.; Liu, F. A Membrane Modified with Nitrogen-Doped TiO2/Graphene Oxide for Improved Photocatalytic Performance. Appl. Sci. 2019, 9, 855. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zhu, Y.; Jiang, Y. Photocatalytic self-cleaning carbon nitride nanotube intercalated reduced graphene oxide membranes for enhanced water purification. Chem. Eng. J. 2019, 356, 915–925. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Ismail, A.; Mohamed, M.A.; Othman, M.; Rahman, M.A.; Othman, N.H.; Nor, N.; Yusof, N.; et al. Mechanistic insight of the formation of visible-light responsive nanosheet graphitic carbon nitride embedded polyacrylonitrile nanofibres for wastewater treatment. J. Water Process Eng. 2020, 33, 101015. [Google Scholar] [CrossRef]
- Wang, S.; Li, F.; Dai, X.; Wang, C.; Lv, X.; Waterhouse, G.I.; Fan, H.; Ai, S. Highly flexible and stable carbon nitride/cellulose acetate porous films with enhanced photocatalytic activity for contaminants removal from wastewater. J. Hazard. Mater. 2020, 384, 121417. [Google Scholar] [CrossRef]
- Nair, A.K.; JagadeeshBabu, P.E. TiO2 nanosheet-graphene oxide based photocatalytic hierarchical membrane for water purification. Surf. Coat. Technol. 2017, 320, 259–262. [Google Scholar] [CrossRef]
- Nair, A.K.; JagadeeshBabu, P. Ag-TiO2 nanosheet embedded photocatalytic membrane for solar water treatment. J. Environ. Chem. Eng. 2017, 5, 4128–4133. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, X.; Dall’Agnese, Y.; Dall’Agnese, C.; Duan, S.; Gao, Y.; Chen, G.; Wang, X.F. 2D MXenes as Co-catalysts in Photocatalysis: Synthetic Methods. Nano-Micro Lett. 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Alam, I.; Guiney, L.M.; Hersam, M.C.; Chowdhury, I. Pressure-driven water transport behavior and antifouling performance of two-dimensional nanomaterial laminated membranes. J. Membr. Sci. 2020, 599, 117812. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Li, J.; Li, J.; Bian, W.; Ji, S. Layer-by-layer self-assembly of polycation/GO nanofiltration membrane with enhanced stability and fouling resistance. Sep. Purif. Technol. 2016, 160, 123–131. [Google Scholar] [CrossRef]
- Vetrivel, S.; Saraswathi, M.S.A.; Rana, D.; Divya, K.; Nagendran, A. Cellulose acetate composite membranes tailored with exfoliated tungsten disulfide nanosheets: Permeation characteristics and antifouling ability. Int. J. Biol. Macromol. 2018, 115, 540–546. [Google Scholar] [CrossRef]
- Saraswathi, M.S.A.; Rana, D.; Melbiah, J.B.; Mohan, D.; Nagendran, A. Effective removal of bovine serum albumin and humic acid contaminants using poly (amide imide) nanocomposite ultrafiltration membranes tailored with GO and MoS2 nanosheets. Mater. Chem. Phys. 2018, 216, 170–176. [Google Scholar] [CrossRef]
- Dong, H.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Clay nanosheets as charged filler materials for high-performance and fouling-resistant thin film nanocomposite membranes. J. Membr. Sci. 2015, 494, 92–103. [Google Scholar] [CrossRef]
- Bi, Q.; Zhang, C.; Liu, J.; Cheng, Q.; Xu, S. A nanofiltration membrane prepared by PDA-C3N4 for removal of divalent ions. Water Sci. Technol. 2020, 81, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Arthanareeswaran, G.; Bose, A.C.; Kumar, P.S. Hydrophilic hierarchical carbon with TiO2 nanofiber membrane for high separation efficiency of dye and oil-water emulsion. Sep. Purif. Technol. 2020, 241, 116709. [Google Scholar] [CrossRef]
- Abdikheibari, S.; Lei, W.; Dumée, L.F.; Barlow, A.J.; Baskaran, K. Novel thin film nanocomposite membranes decorated with few-layered boron nitride nanosheets for simultaneously enhanced water flux and organic fouling resistance. Appl. Surf. Sci. 2019, 488, 565–577. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasool, K.; Madhavan, V.E.; Aïssa, B.; Gogotsi, Y.; Mahmoud, K.A. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533. [Google Scholar] [CrossRef]
- Liu, G.; Han, K.; Zhou, Y.; Ye, H.; Zhang, X.; Hu, J.; Li, X. Facile Synthesis of Highly Dispersed Ag Doped Graphene Oxide/Titanate Nanotubes as a Visible Light Photocatalytic Membrane for Water Treatment. ACS Sustain. Chem. Eng. 2018, 6, 6256–6263. [Google Scholar] [CrossRef]
- Xu, H.; Ding, M.; Chen, W.; Li, Y.; Wang, K. Nitrogen–doped GO/TiO2 nanocomposite ultrafiltration membranes for improved photocatalytic performance. Sep. Purif. Technol. 2018, 195, 70–82. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Chen, J.; Cui, L.; Jing, W. Solvothermal-induced assembly of 2D-2D rGO-TiO2 nanocomposite for the construction of nanochannel membrane. J. Membr. Sci. 2020, 600, 117870. [Google Scholar] [CrossRef]
- Ghalamchi, L.; Aber, S.; Vatanpour, V.; Kian, M. Comparison of NLDH and g-C3N4 nanoplates and formative Ag3PO4 nanoparticles in PES microfiltration membrane fouling: Applications in MBR. Chem. Eng. Res. Des. 2019, 147, 443–457. [Google Scholar] [CrossRef]
- Eke, J.; Elder, K.; Escobar, I. Self-Cleaning Nanocomposite Membranes with Phosphorene-Based Pore Fillers for Water Treatment. Membranes 2018, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Abdikheibari, S.; Lei, W.; Dumée, L.F.; Milne, N.; Baskaran, K. Thin film nanocomposite nanofiltration membranes from amine functionalized-boron nitride/polypiperazine amide with enhanced flux and fouling resistance. J. Mater. Chem. A 2018, 6, 12066–12081. [Google Scholar] [CrossRef]
- Abdikheibari, S.; Dumée, L.F.; Jegatheesan, V.; Mustafa, Z.; Le-Clech, P.; Lei, W.; Baskaran, K. Natural organic matter removal and fouling resistance properties of a boron nitride nanosheet-functionalized thin film nanocomposite membrane and its impact on permeate chlorine demand. J. Water Process Eng. 2020, 34, 101160. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, M.; Hou, J.; Wang, J.; Lin, J.; Zhang, Y.; Liu, J.; der Bruggen, B.V. Surface zwitterionic functionalized graphene oxide for a novel loose nanofiltration membrane. J. Mater. Chem. A 2016, 4, 1980–1990. [Google Scholar] [CrossRef]
- Wang, X.; Feng, M.; Liu, Y.; Deng, H.; Lu, J. Fabrication of graphene oxide blended polyethersulfone membranes via phase inversion assisted by electric field for improved separation and antifouling performance. J. Membr. Sci. 2019, 577, 41–50. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Z.; Tai, M.; Sun, D.D.; Ng, W. Multifunctional graphene oxide–TiO2 microsphere hierarchical membrane for clean water production. Appl. Catal. B 2013, 138–139, 17–25. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, G.; Han, K.; Ye, H.; Wei, S.; Zhou, Y. One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: Crossflow filtration operation and membrane fouling analysis. Chem. Eng. Process. Process Intensif. 2017, 120, 20–26. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Y.; Zhu, J. Photocatalytic Antifouling Graphene Oxide-Mediated Hierarchical Filtration Membranes with Potential Applications on Water Purification. ACS Appl. Mater. Interfaces 2014, 6, 16117–16123. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Z.; Zhai, D.; Liu, Y.; Liu, Q.; Xue, L.; Gao, C. Dye Degrading and Fouling-Resistant Membranes Formed by Deposition with Ternary Nanocomposites of N-Doped Graphene/TiO2/Activated Carbon. Membranes 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Han, K.; Ye, H.; Zhu, C.; Gao, Y.; Liu, Y.; Zhou, Y. Graphene oxide/triethanolamine modified titanate nanowires as photocatalytic membrane for water treatment. Chem. Eng. J. 2017, 320, 74–80. [Google Scholar] [CrossRef]
- Yu, Z.; Feng, X.; Min, X.; Li, X.; Shao, L.; Zeng, H. RGO/PDA/Bi12O17Cl2–TiO2 composite membranes based on Bi12O17Cl2–TiO2 heterojunctions with excellent photocatalytic activity for photocatalytic dyes degradation and oil–water separation. J. Mater. Sci. Mater. Electron. 2019, 30, 18246–18258. [Google Scholar] [CrossRef]
- Shahabi, S.S.; Azizi, N.; Vatanpour, V.; Yousefimehr, N. Novel functionalized graphitic carbon nitride incorporated thin film nanocomposite membranes for high-performance reverse osmosis desalination. Sep. Purif. Technol. 2020, 235, 116134. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, L.; Meng, M.; Zhang, Q.; Li, B.; Wu, Y.; Zhang, Y.; Lang, J.; Li, C. Facile preparation of antifouling g-C3N4/Ag3PO4 nanocomposite photocatalytic polyvinylidene fluoride membranes for effective removal of rhodamine B. Korean J. Chem. Eng. 2019, 36, 236–247. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Gao, Y.; Shu, L. Ag modified g-C3N4 composite entrapped PES UF membrane with visible-light-driven photocatalytic antifouling performance. RSC Adv. 2017, 7, 42919–42928. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Meng, M.; Cui, Y.; Wu, Y.; Zhang, Y.; Dong, H.; Zhu, Z.; Feng, Y.; Wu, C. Changing conventional blending photocatalytic membranes (BPMs): Focus on improving photocatalytic performance of Fe3O4/g-C3N4/PVDF membranes through magnetically induced freezing casting method. Chem. Eng. J. 2019, 365, 405–414. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Cheng, W.; Hu, J.; Shi, L.; Yi, K. Evaluation of self-cleaning performance of the modified g-C3N4 and GO based PVDF membrane toward oil-in-water separation under visible-light. Chemosphere 2019, 230, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yu, Z.; Li, F.; Chen, Q.; Yin, D.; Min, X. A novel reduced graphene oxide-based composite membrane prepared via a facile deposition method for multifunctional applications: Oil/water separation and cationic dyes removal. Sep. Purif. Technol. 2018, 200, 130–140. [Google Scholar] [CrossRef]
- Zhan, Y.; He, S.; Wan, X.; Zhao, S.; Bai, Y. Thermally and chemically stable poly(arylene ether nitrile)/halloysite nanotubes intercalated graphene oxide nanofibrous composite membranes for highly efficient oil/water emulsion separation in harsh environment. J. Membr. Sci. 2018, 567, 76–88. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, M.; Zhang, W.; Cao, Y.; Chen, Y.; Lin, X.; Xu, L.; Li, C.; Feng, L.; Wei, Y. Ultralight free-standing reduced graphene oxide membranes for oil-in-water emulsion separation. J. Mater. Chem. A 2015, 3, 20113–20117. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, D.; Zhao, J.; Feng, Q.; Li, Z.; Bai, H.; Sun, D.D. Efficient Oil/Water Separation Membrane Derived from Super-Flexible and Superhydrophilic Core–Shell Organic/Inorganic Nanofibrous Architectures. Polymers 2019, 11, 974. [Google Scholar] [CrossRef]
- Saththasivam, J.; Wang, K.; Yiming, W.; Liu, Z.; Mahmoud, K.A. A flexible Ti3C2Tx (MXene)/paper membrane for efficient oil/water separation. RSC Adv. 2019, 9, 16296–16304. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zeng, H.; Min, X.; Zhu, X. High-performance composite photocatalytic membrane based on titanium dioxide nanowire/graphene oxide for water treatment. J. Appl. Polym. Sci. 2019, 137, 48488. [Google Scholar] [CrossRef]
- Nishimoto, S.; Tomoishi, S.; Kameshima, Y.; Fujii, E.; Miyake, M. Self-cleaning efficiency of titanium dioxide surface under simultaneous UV irradiation of various intensities and water flow. J. Ceram. Soc. Jpn. 2014, 122, 513–516. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.R.; Tadepalli, S.; Nergiz, S.Z.; Liu, K.K.; You, L.; Tang, Y.; Singamaneni, S.; Jun, Y.S. Hydrophilic, Bactericidal Nanoheater-Enabled Reverse Osmosis Membranes to Improve Fouling Resistance. ACS Appl. Mater. Interfaces 2015, 7, 11117–11126. [Google Scholar] [CrossRef]
- Cao, B.; Ansari, A.; Yi, X.; Rodrigues, D.F.; Hu, Y. Gypsum scale formation on graphene oxide modified reverse osmosis membrane. J. Membr. Sci. 2018, 552, 132–143. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Zouari, N. Functionalization of reverse osmosis membrane with graphene oxide and polyacrylic acid to control biofouling and mineral scaling. Sci. Total Environ. 2020, 736, 139500. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Zouari, N. Functionalization of reverse osmosis membrane with graphene oxide to reduce both membrane scaling and biofouling. Carbon 2020, 166, 374–387. [Google Scholar] [CrossRef]











| Nanosheet | Type | Materials | Foulant | WCA | Highlights | Ref. |
|---|---|---|---|---|---|---|
| Normal Testing | ||||||
| GO | Stacked | PDA(C), halloysite-NT, PEN(S) | n-hexane-in-water emulsion | 0 | OCA = 136 ± 2, stable at high temperatures, electrospun support | [226] |
| GO | MMM | PVA, PES(S) | surfactant/sunflower oil and olive oil mixture | 30.5 ± 3.3 | OCA = 141.6 ± 3.5 | [130] |
| rGO | Stacked | PDA(C), MCE(S) | 1,2-dichlorethane, toluene, n-hexane, diesel | near 0 | OCA = 156.1±1.2 | [227] |
| rGO | Stacked | PDA(C), SiO2-NP, PVDF(S) | diesel oil/water emulsion | 0 | OCA = 130, FRR = 87.2% | [225] |
| CuO | MMM | PVDF-HFP(S) | olive oil, cooking oil, lubricant oil | 0 | OCA = 152.4, electronspun polymer with nanosheet shell | [228] |
| Ti3C2Tx | Stacked | white print paper(S) | sunflower oil, diesel oil, silicon oil, petroleum ether, hexane | 0 | OCA = 137 | [229] |
| Photo-Assisted Testing | ||||||
| GO(CC) | Stacked | PDA(C), TiO2-NW(P), CA(S) | MB and diesel oil/gasoline/ dichloro–methane–water emulsion | visible light, OCA = 132 | [230] | |
| rGO(CC), Bi12O17Cl2(P) | Stacked | PDA(C), TiO2-NW(CC), CA(S) | MB and diesel oil/water emulsion | visible light | [219] | |
| GO(CC), gCN(H)(CC) | Stacked | TiO2-NP(P) | soy-bean oil | 43 | visible light, OCA = 170, FRR = 95% | [155] |
| GO(C), MCU-CN(H)(P) | Stacked | GA(C), PVDF(S) | SDS-diesel oil /petroleum-ether/ dichloromethane/hexane in water | visible light | [224] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loske, L.; Nakagawa, K.; Yoshioka, T.; Matsuyama, H. 2D Nanocomposite Membranes: Water Purification and Fouling Mitigation. Membranes 2020, 10, 295. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10100295
Loske L, Nakagawa K, Yoshioka T, Matsuyama H. 2D Nanocomposite Membranes: Water Purification and Fouling Mitigation. Membranes. 2020; 10(10):295. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10100295
Chicago/Turabian StyleLoske, Lara, Keizo Nakagawa, Tomohisa Yoshioka, and Hideto Matsuyama. 2020. "2D Nanocomposite Membranes: Water Purification and Fouling Mitigation" Membranes 10, no. 10: 295. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10100295