Synthesis of Silica Membranes by Chemical Vapor Deposition Using a Dimethyldimethoxysilane Precursor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Fabrication
2.2.1. Preparation of the Dipping Sols
2.2.2. Preparation of the Intermediate Layers
2.2.3. Preparation of the Topmost Silica Layers
2.3. Evaluation of the As-Synthesized Membranes
2.3.1. SEM Analysis
2.3.2. Single Gas Permeation Test
2.3.3. Hydrothermal Stability Test
3. Results and Discussion
3.1. Morphology and Structure of the Membranes
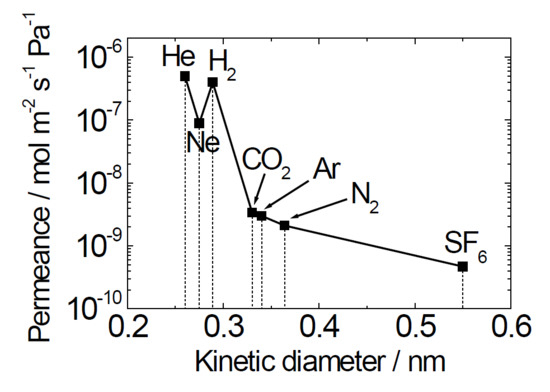
3.2. Gas Permeation Properties of the Membranes
3.3. Hydrothermal Stability of the Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint Annaland, M. Review Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T. Review of silica membranes for hydrogen separation prepared by CVD. Sep. Purif. Technol. 2013, 111, 20–42. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T.; de Souza, K.R.; Noronha, F.B. Review of silica membranes for hydrogen separation prepared by chemical vapor deposition. In Inorganic, Polymeric and Composite Membranes, Structure, Function and Correlations, Membrane Science and Technology Series; Oyama, S.T., Stagg-Williams, S.M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2011; Volume 14, p. 25. [Google Scholar]
- Ten Hove, M.; Luiten-Olieman, M.W.J.; Huiskes, C.; Nijmeijer, A.; Winnubst, L. Hydrothermal stability of silica, hybrid silica and Zr-doped hybrid silica membranes. Sep. Purif. Technol. 2017, 189, 48–53. [Google Scholar] [CrossRef]
- Akamatsu, K.; Tago, T.; Seshimo, M.; Nakao, S.-I. Long-term stable H2 production from methylcyclohexane using a membrane reactor with a dimethoxydiphenylsilane-derived silica membrane prepared via chemical vapor deposition. Ind. Eng. Chem. Res. 2015, 54, 3996–4000. [Google Scholar] [CrossRef]
- Nagasawa, H.; Minamizawa, T.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Microporous organosilica membranes for gas separation prepared via PECVD using different O/Si ratio precursors. J. Membr. Sci. 2015, 489, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Diniz da Costa, J.C.; Bhatia, S.K. Adsorption and transport of gases in a supported microporous silica membrane. J. Membr. Sci. 2015, 460, 46–61. [Google Scholar] [CrossRef]
- Yin, Y.; Xua, T.; Shen, X.; Wu, H.; Jiang, Z. Fabrication of chitosan/zwitterion functionalized titania–silica hybrid membranes with improved proton conductivity. J. Membr. Sci. 2014, 469, 355–363. [Google Scholar] [CrossRef]
- Okubo, T.; Inoue, H. Introduction of specific gas reactivity to porous glass membranes by treatment with tetraethoxysilane. J. Membr. Sci. 1989, 42, 109–119. [Google Scholar] [CrossRef]
- Gavalas, G.R.; Megiris, C.E.; Nam, S.W. Deposition of H2-permselective SiO2 films. Chem. Eng. Sci. 1989, 44, 1829–1839. [Google Scholar] [CrossRef]
- De Lange, R.S.A.; Keizer, K.; Burggraaf, A.J. Analysis and theory of gas transport in microporous sol-gel derived ceramic membranes. J. Membr. Sci. 1995, 104, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Okubo, T.; Haruta, K.; Kusakabe, K.; Morooka, S.; Anzai, H.; Akiyama, S. Preparation of sol-gel derived thin membrane on a porous ceramic hollow filter by the filtration technique. J. Membr. Sci. 1991, 59, 73–83. [Google Scholar] [CrossRef]
- Nam, S.W.; Ha, H.Y.; Yoon, S.P.; Han, J.; Lim, T.H.; Oh, I.H.; Hong, S.A. Hydrogen-permselective TiO2/SiO2 membranes formed by chemical vapor deposition. Korean Membr. J. 2001, 3, 69–78. [Google Scholar]
- Megiris, C.E.; Glezer, J.H.E. Permeation of silicon dioxide films by low-pressure chemical vapor deposition on dense and porous alumina substrates. Chem. Eng. Sci. 1992, 47, 3925–3933. [Google Scholar] [CrossRef]
- Ngamou, P.; Overbeek, J.; Kreiter, R.; van Veen, H.; Vente, J.; Wienk, I.; Cuperus, P.; Creatore, M. Plasma deposited hybrid silica membranes with a controlled retention of organic bridges. J. Mater. Chem. A 2013, 1, 5567–5576. [Google Scholar] [CrossRef] [Green Version]
- Walkiewicz-Pietrzykowska, A.; Cotrino, J.; Gonzalez-Elipe, A.R. Deposition of thin films of SiOxCyH in a surfatron microwave plasma reactor with hexamethyldisiloxane as precursor. Chem. Vap. Depos. 2005, 11, 317–323. [Google Scholar] [CrossRef]
- Nomura, M. Stability of H2-permselective SiO2 films formed by chemical vapor deposition. Membrane 2007, 32, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yamada, H.; Saito, T.; Kai, T.; Murakami, K.; Nakashima, M.; Ohshita, J.; Akamatsu, K.; Nakao, S. Development of hydrogen-selective triphenylmethoxysilane-derived silica membranes with tailored pore size by chemical vapor deposition. J. Membr. Sci. 2016, 499, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Niimi, T.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Ito, K.; Tsuru, T. Preparation of BTESE-derived organosilica membranes for catalytic membrane reactors of methylcyclohexane dehydrogenation. J. Membr. Sci. 2014, 455, 375–383. [Google Scholar] [CrossRef]
- Lee, H.R.; Kanezashi, M.; Shimomura, Y.; Yoshioka, T.; Tsuru, T. Evaluation and fabrication of pore-size-tuned silica membranes with tetraethoxydimethyl disiloxane for gas separation. AIChE J. 2011, 57, 2755–2765. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ying, X.; Tokimasa, Y.; Nair, B.N.; Sugawara, T.; Nakao, S.I. Reaction control of tetraethylorthosilicate (TEOS)/O3 and tetramethylorthosilicate (TMOS)/O3 counter diffusuin chemical vapour deposition for preparation of molecular-sieve membranes. Phys. Chem. Chem. Phys. 2000, 2, 4465–4475. [Google Scholar] [CrossRef]
- Kageyama, N.; Sugawara, T.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Overcoming pressure drop losses in membrane reactors by semi-batch operation. Sep. Purif. Tech. 2017, 185, 175–185. [Google Scholar] [CrossRef]
- Hekkink, J.H.A.; de Lange, R.S.A.; Ten Hoeve, A.A.; Blankenvoorde, P.J.A.M.; Keizer, K.; Burggraaf, A.J. Characterization and permeation properties of binary SiO2-TiO2 and SiO2-Al2O3 modified gamma-alumina membranes. Key Eng. Mater. 1991, 61, 375–378. [Google Scholar]
- Yoshida, K.; Hirano, Y.; Fujii, H.; Tsuru, T.; Asaeda, M. Hydrothermal stability and performance of silica-zirconia membranes for hydrogen separation in hydrothermal conditions. J. Chem. Eng. Jpn. 2001, 34, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Organic-inorganic hybrid silica membranes with controlled silica network size: Preparation and gas permeation characteristics. J. Membr. Sci. 2010, 348, 310–318. [Google Scholar] [CrossRef]
- Li, H.; Schygulla, U.; Hoffmann, J.; Nichoff, P.; Haas-Santo, K.; Dittmeyer, R. Experimental and modeling study of gas transport through composite ceramic membranes. Chem. Eng. Sci. 2014, 108, 94–102. [Google Scholar] [CrossRef]
- Oyama, S.T.; Lee, D.; Hacarlioglu, P.; Saraf, R.F. Theory of hydrogen permeability in nonporous silica membranes. J. Membr. Sci. 2004, 244, 45–53. [Google Scholar] [CrossRef]
- Gu, Y.; Oyama, S.T. Ultrathin, hydrogen-selective silica membranes deposited on alumina-graded structures prepared from size-controlled boehmite sols. J. Membr. Sci. 2007, 306, 216–227. [Google Scholar] [CrossRef]
- Mise, Y.; Ahn, S.-J.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Fabrication and evaluation of trimethylmethoxysilane (TMMOS)-derived membranes for gas separation. Membranes 2019, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Lundin, S.-T.B.; Ahn, S.-J.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Gas separation silica membranes prepared by chemical vapor deposition of methyl-substituted silanes. Membranes 2019, 9, 144. [Google Scholar] [CrossRef] [Green Version]
- Oyama, S.T.; Yamada, M.; Sugawara, T.; Takagaki, A.; Kikuchi, R. Review on mechanisms of gas permeation through inorganic membranes. J. Jpn. Pet. Inst. 2011, 54, 298–309. [Google Scholar] [CrossRef]
- Igi, R.; Kanezashi, M.; Yoshioka, T.; Fujisaki, S.; Iwamoto, Y.; Tsuru, T. Permeation properties of hydrogen and water vapor through porous silica membranes at high temperatures. AIChE J. 2011, 57, 618–629. [Google Scholar]
- Kanezashi, M.; Sasaki, T.; Tawarayama, H.; Nagasawa, H.; Yoshioka, T.; Ito, K.; Tsuru, T. Experimental and theoretical study on small gas permeation properties through amorphous silica membranes fabricated at different temperatures. J. Phys. Chem. 2014, 118, 20323–20331. [Google Scholar] [CrossRef]
- Okubo, T.; Watanabe, M.; Kusakabe, K.; Morooka, S. Preparation of γ-alumina thin membrane by sol-gel processing and its characterization by gas permeation. J. Mater. Sci. 1990, 25, 4822–4827. [Google Scholar] [CrossRef]
- Gu, Y.; Oyama, S.T. High molecular permeance in a poreless ceramic membrane. Adv. Mater. 2007, 19, 1636–1640. [Google Scholar] [CrossRef]
- Ahn, S.J.; Yun, G.N.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Effects of pressure contact time, permeance, and selectivity in membrane reactors: The case of the dehydrogenation of ethane. Sep. Purif. Technol. 2018, 194, 197–206. [Google Scholar] [CrossRef]
- Ma, L.-C.; Castro-Dominguez, B.; Kazantzis, N.K.; Ma, Y.H. A cost assessment study for a large-scale water gas shift catalytic membrane reactor module in the presence of uncertainty. Sep. Purif. Technol. 2016, 166, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Yu, X.; Niimi, T.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Methylcyclohexane dehydrogenation for hydrogen production via a bimodal catalytic membrane reactor. AIChE J. 2015, 61, 1628–1638. [Google Scholar] [CrossRef]
- Araki, S.; Miyanishi, H.; Yano, H.; Tanaka, S.; Miyake, Y. Water gas shift reaction in a membrane reactor using a high hydrogen permselective silica membrane. Sep. Sci. Technol. 2013, 48, 76–83. [Google Scholar] [CrossRef]
- Lim, H.; Gu, Y.; Oyama, S.T. Studies of the effect of pressure and hydrogen permeance on the ethanol steam reforming reaction with palladium- and silica-based membranes. J. Membr. Sci. 2012, 396, 119–127. [Google Scholar] [CrossRef]
- Nagano, T.; Sato, K. Degradation mechanism of an H2-permselective amorphous silica membrane. J. Mater. Sci. 2014, 49, 4115–5120. [Google Scholar] [CrossRef]
- Nomura, M.; Aida, H.; Gopalakrishnan, S.; Sugawara, T.; Nakao, S.I.; Yamazaki, S.; Inada, T.; Iwamoto, Y. Steam stability of a silica membrane prepared by counter diffusion chemical vapor deposition. Desalination 2006, 193, 1–7. [Google Scholar] [CrossRef]
- Lim, H.; Gu, Y.; Oyama, S.T. Reaction of primary and secondary products in a membrane reactor: Studies of ethanol steam reforming with a silica-alumina composite membrane. J. Membr. Sci. 2010, 351, 149–159. [Google Scholar] [CrossRef]
- Kageyama, N.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Synthesis and characterization of a silica-alumina composite membrane and its application in a membrane reactor. Sep. Purif. Technol. 2018, 195, 437–445. [Google Scholar] [CrossRef]
- Gu, Y.; Hacarlioglu, P.; Oyama, S.T. Hydrothermally stable silica–alumina composite membranes for hydrogen separation. J. Membr. Sci. 2008, 310, 28–37. [Google Scholar] [CrossRef]
- Gu, Y.; Oyama, S.T. Permeation properties and hydrothermal stability of silica-titania membranes supported on porous alumina substrates. J. Membr. Sci. 2009, 345, 267–275. [Google Scholar] [CrossRef]
- Sea, B.K.; Soewito, E.; Watanabe, M.; Kusakabe, K.; Morooka, S.; Kim, S.S. Hydrogen recovery from a H2–H2O–HBr mixture utilizing silica-based membranes at elevated temperatures. 1. Preparation of H2O- and H2-selective membranes. Ind. End. Chem. Res. 1998, 37, 2502–2508. [Google Scholar] [CrossRef]
- Akamatsu, K.; Nakane, M.; Sugawara, T.; Nakao, S.I. Performance under thermal and hydrothermal condition of amorphous silica membrane prepared by chemical vapor deposition. AIChE J. 2009, 55, 2197–2203. [Google Scholar] [CrossRef]
- Nagano, T.; Sato, K.; Saito, T.; Takahashi, S. Hydrothermal stability of mesoporous Ni-doped γ-Al2O3. J. Ceram. Soc. Jpn. 2009, 117, 832–835. [Google Scholar] [CrossRef] [Green Version]
- Ohta, Y.; Akamatsu, K.; Sugawara, T.; Miyoshi, A.; Nakao, S. Development of pore-size-controlled silica membranes for gas separation by chemical vapor deposition. J. Membr. Sci. 2008, 315, 93–99. [Google Scholar] [CrossRef]
- Seshimo, M.; Saito, T.; Akamatsu, K.; Segawa, A.; Nakao, S. Influence of toluene vapor on the H2-selective performance of dimethoxy diphenylsilane-derived silica membranes prepared by the chemical vapor deposition method. J. Membr. Sci. 2012, 415–416, 51–56. [Google Scholar] [CrossRef]
- Seshimo, M.; Akamatsu, K.; Furuta, S.; Nakao, S. H2 purification durability of dimethoxy diphenylsilane-derived silica membranes with H2-toluene mixtures. Ind. Eng. Chem. Res. 2013, 52, 17257–17262. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Yun, G.-N.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Synthesis and characterization of hydrogen selective silica membrane prepared by chemical vapor deposition of vinyltriethoxysilane. J. Membr. Sci. 2018, 550, 1–8. [Google Scholar] [CrossRef]
- Nishida, R.; Tago, T.; Saitoh, T.; Seshimo, M.; Nakao, S.-I. Development of CVD silica membranes having high hydrogen permeance and steam durability and a membrane reactor for a water gas shift reaction. Membranes 2019, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawamura, K.-I.; Okamoto, S.; Todokoro, Y. Development of mass production technology of highly permeable nano-porous supports for silica-based separation membranes. Membranes 2019, 9, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, K.; Ikeda, A.; Takeuchi, T.; Yoshiura, J.; Nomura, M. Silica-based RO membranes for separation of acidic solution. Membranes 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, M.; Nishi, Y.; Sakanishi, T.; Utsumi, K.; Nakamura, R. Preparation of thin LiSiO membranes by using a CVD method. Energy Procedia 2013, 37, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Oyama, S.T.; Hacarlioglu, P. The boundary between simple and complex descriptions of membrane reactors: The transition between 1-D and 2-D analysis. J. Membr. Sci 2009, 337, 188–199. [Google Scholar] [CrossRef]
- Oyama, S.T.; Lee, D.; Sugiyama, S.; Fukui, K.; Iwasawa, Y. Characterization of a highly selective hydrogen permeable silica membrane. J. Mater. Sci. 2001, 36, 5213–5223. [Google Scholar] [CrossRef]
- Belhaj Messaoud, S.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Alkylamine-silica hybrid membranes for carbon dioxide/methane separation. J. Membr. Sci. 2015, 477, 161–171. [Google Scholar] [CrossRef]
- Kanezashi, M.; Sasaki, T.; Tawarayama, H.; Yoshioka, T.; Tsuru, T. Hydrogen permeation properties and hydrothermal stability of sol-gel-derived amorphous silica membranes fabricated at high temperatures. J. Am. Ceram. Soc. 2013, 96, 2950–2957. [Google Scholar] [CrossRef]














| Samples before CVD | Samples after CVD | |
|---|---|---|
| Surface | ∙ Particle size of γ-alumina ∙ Porosity ∙ Smoothness ∙ Presence of pinholes | ∙ Smoothness ∙ Presence of pinholes |
| Cross-section | ∙ Thickness of the γ-alumina layer ∙ Presence of γ-alumina particle ∙ infiltration into α-alumina ∙ Presence of cracks | ∙ Thickness of the silica layer ∙ Presence of silica infiltration ∙ into γ-alumina ∙ Presence of cracks |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyama, S.T.; Aono, H.; Takagaki, A.; Sugawara, T.; Kikuchi, R. Synthesis of Silica Membranes by Chemical Vapor Deposition Using a Dimethyldimethoxysilane Precursor. Membranes 2020, 10, 50. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10030050
Oyama ST, Aono H, Takagaki A, Sugawara T, Kikuchi R. Synthesis of Silica Membranes by Chemical Vapor Deposition Using a Dimethyldimethoxysilane Precursor. Membranes. 2020; 10(3):50. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10030050
Chicago/Turabian StyleOyama, S. Ted, Haruki Aono, Atsushi Takagaki, Takashi Sugawara, and Ryuji Kikuchi. 2020. "Synthesis of Silica Membranes by Chemical Vapor Deposition Using a Dimethyldimethoxysilane Precursor" Membranes 10, no. 3: 50. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10030050