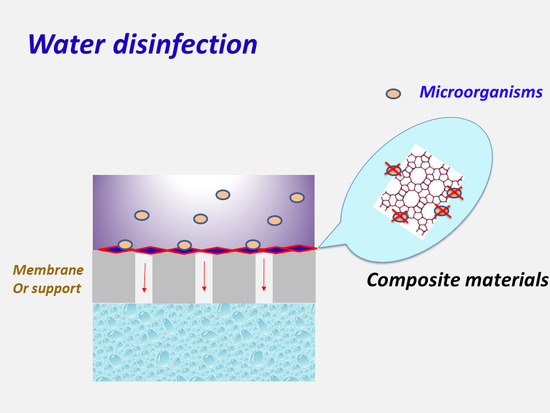
The Role of New Inorganic Materials in Composite Membranes for Water Disinfection
Abstract
:1. Introduction
2. Composite Membranes for Water Disinfection: The Role of New Inorganic Materials
3. Concluding Remarks and Suggestions for the New Readers in the Field
- It is needed to develop low-cost composite materials and membranes. In general, the membranes, as well as the primary reactive materials of the composites, represent the main cost of the overall disinfection processes.
- Commonly, the synthesis procedures and techniques for new hybrid composites are generally implemented at a lab-scale. Based on the excellent antibacterial performance, researchers should start to implement and develop protocols for the preparation of large-scale composites and membranes.
- To date, most of the studies have evaluated the disinfection activity of composite membranes and composite materials using model water solutions; it is recommended to start the testing of hybrid composites using real complex aqueous solutions since few reports have used natural complex water systems (e.g., natural water coming from rivers, lakes, and taps) [38,43,76].
- It is recommended to evaluate the reusability of new hybrid composites after testing their antibacterial activity and pollutant removal. There are few studies addressing the evaluation of the reuse of such materials [12,88]. In this way, the overall disinfection cost could be reduced. Moreover, there is also a big need for developing long-term operation tests to analyze the stability of such new materials.
- The new researchers in the field should also initiate the exploration of other biopolymers in the preparation of composite membranes. Today, there is a trend of using more environmentally friendly materials in accordance with the current environmental regulations.
- Finally, the mechanism of action of the different composite materials has been given. At this point, new researchers aiming for the development of new composites should provide a good understanding of the action of the developed hybrid materials.
Funding
Acknowledgments
Conflicts of Interest
References
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Castro-Muñoz, R. Retention profile on the physicochemical properties of maize cooking by-product using a tight ultrafiltration membrane. Chem. Eng. Commun. 2019, 1–9. [Google Scholar] [CrossRef]
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Piá, A.; Alcaina-Miranda, M.I.; Dhahbi, M. Reactive dyes rejection and textile effluent treatment study using ultrafiltration and nanofiltration processes. Desalination 2012, 297, 87–96. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.; Figoli, A. Progress of Nanocomposite Membranes for Water Treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argun, M.S.; Argun, M.E. Treatment and alternative usage possibilities of a special wastewater: Nejayote. J. Food Process Eng. 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Yanez-Fernandez, J. Valorization of Nixtamalization wastewaters (Nejayote) by integrated membrane process. Food Bioprod. Process. 2015, 95, 7–18. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Yanez-Fernandez, J. The Use of Nixtamalization Waste Waters Clarified by Ultrafiltration for Production of a Fraction Rich in Phenolic Compounds. Waste Biomass Valorization 2016, 7, 1167–1176. [Google Scholar] [CrossRef]
- Manju, S.; Sagar, N. Renewable energy integrated desalination: A sustainable solution to overcome future fresh-water scarcity in India. Renew. Sustain. Energy Rev. 2017, 73, 594–609. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Rodríguez-Romero, V.; Yáñez-Fernández, J.; Fíla, V. Water production from food processing wastewaters by integrated membrane systems: Sustainable approach. Water Technol. Sci. 2017, 8, 129–136. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Fíla, V.; Denis, P.C.; Ruby-Figueroa, R. Current Role of Membrane Technology: From the Treatment of Agro-Industrial by-Products up to the Valorization of Valuable Compounds. Waste Biomass Valorization 2017, 9, 513–529. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Murali, R.; Mary, R.R.; Thanikaivelan, P.; Sekaran, G.; Villalan, A.; Ragothaman, M.; Rathinasamy, R.M.; Palanisamy, T.; et al. Prodigiosin–Iron-Oxide–Carbon Matrix for Efficient Antibiotic-Resistant Bacterial Disinfection of Contaminated Water. ACS Sustain. Chem. Eng. 2019, 7, 3164–3175. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B.; Boyce, J. The World Health Organization Guidelines on Hand Hygiene in Health Care and Their Consensus Recommendations. Infect. Control. Hosp. Epidemiol. 2009, 30, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Buonomenna, M.G. Smart Composite Membranes for Advanced Wastewater Treatments; Elsevier Ltd: Amsterdam, The Netherlands, 2016; pp. 371–419. [Google Scholar]
- Pichardo-Romero, D.; Garcia-Arce, Z.; Zavala-Ramírez, A.; Castro-Muñoz, R. Current Advances in Biofouling Mitigation in Membranes for Water Treatment: An Overview. Processes 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, M.; Fang, F.; Cui, L.; Wu, J.; Field, R.W.; Zhang, K. Biogenic silver nanocomposite TFC nanofiltration membrane with antifouling properties. Desalin. Water Treat. 2015, 57, 10560–10571. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, O.S.; Molefe, N.I.; Awakan, O.; Nwonuma, C.O.; Alejolowo, O.O.; Olaolu, T.; Maimako, R.F.; Suganuma, K.; Han, Y.; Kato, K. Metal nanoparticles restrict the growth of protozoan parasites. Artif. Cells Nanomed. Biotechnol. 2018, 46, S86–S94. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, O.S.; Whiteley, C. Interaction of metal nanoparticles with recombinant arginine kinase from Trypanosoma brucei: Thermodynamic and spectrofluorimetric evaluation. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorozynski, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. ChemInform Abstract: Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 44, 1636–1653. [Google Scholar] [CrossRef]
- Sile-Yuksel, M.; Tas, B.; Koseoglu-Imer, D.Y.; Koyuncu, I. Effect of silver nanoparticle (AgNP) location in nanocomposite membrane matrix fabricated with different polymer type on antibacterial mechanism. Desalination 2014, 347, 120–130. [Google Scholar] [CrossRef]
- Jiang, T.; Feng, L.; Wang, Y. Effect of alginate/nano-Ag coating on microbial and physicochemical characteristics of shiitake mushroom (Lentinus edodes) during cold storage. Food Chem. 2013, 141, 954–960. [Google Scholar] [CrossRef]
- Cao, X.; Tang, M.; Liu, F.; Nie, Y.; Zhao, C. Immobilization of silver nanoparticles onto sulfonated polyethersulfone membranes as antibacterial materials. Colloids Surf. B Biointerfaces 2010, 81, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.A.; Larrea, M.; Tamayo, L.; Rabagliati, F.M.; Azócar, M.I.; Páez, M. Polyethylene/silver-nanofiber composites: A material for antibacterial films. Mater. Sci. Eng. C 2016, 69, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Shan, G.; Wang, C.; Du, J.; Wang, S.; Li, Y.; Chen, X.; Jing, X.; Wei, Y. Preparation of silver nanoparticles dispersed in polyacrylonitrile nanofiber film spun by electrospinning. Mater. Lett. 2005, 59, 3046–3049. [Google Scholar] [CrossRef]
- López-Heras, M.; Theodorou, I.G.; Leo, B.F.; Ryan, M.P.; Porter, A.E. Towards understanding the antibacterial activity of Ag nanoparticles: Electron microscopy in the analysis of the materials-biology interface in the lung. Environ. Sci. Nano 2015, 2, 312–326. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2014, 20, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, H.-H.; Zhuang, G.-L.; Su, Y.-C. The effect of blending ratio on the compatibility, morphology, thermal behavior and pure water permeation of asymmetric CAP/PVDF membranes. Desalination 2012, 284, 269–278. [Google Scholar] [CrossRef]
- Gogoi, S.K.; Gopinath, P.; Paul, A.; Ramesh, A.; Ghosh, S.S.; Chattopadhyay, A. Green Fluorescent Protein-ExpressingEscherichiacolias a Model System for Investigating the Antimicrobial Activities of Silver Nanoparticles. Langmuir 2006, 22, 9322–9328. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Muñoz, R.; Ahmad, M.Z.; Fíla, V. Tuning of Nano-Based Materials for Embedding Into Low-Permeability Polyimides for a Featured Gas Separation. Front. Chem. 2020, 7, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, X.; Lu, J.; Wang, Y.; Li, G.; Zhao, F. Anti-bacterial properties of ultrafiltration membrane modified by graphene oxide with nano-silver particles. J. Colloid Interface Sci. 2016, 484, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.F.; Martinez, D.; Meira, S.M.M.; De Moraes, A.C.M.; Brandelli, A.; Filho, A.G.D.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Zhang, D.; Qi, P. Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J. Colloid Interface Sci. 2011, 360, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Pramanik, A.; Gates, K.; Gao, Y.; Zhang, Q.; Han, F.X.; Begum, S.; Rightsell, C.; Sardar, D.; Ray, P.C. Composites Composed of Polydopamine Nanoparticles, Graphene Oxide, and ϵ-Poly-L-lysine for Removal of Waterborne Contaminants and Eradication of Superbugs. ACS Appl. Nano Mater. 2019, 2, 3339–3347. [Google Scholar] [CrossRef]
- Jones, S.; Pramanik, A.; Kanchanapally, R.; Nellore, B.P.V.; Begum, S.; Sweet, C.; Ray, P.C. Multifunctional Three-Dimensional Chitosan/Gold Nanoparticle/Graphene Oxide Architecture for Separation, Label-Free SERS Identification of Pharmaceutical Contaminants, and Effective Killing of Superbugs. ACS Sustain. Chem. Eng. 2017, 5, 7175–7187. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Zhang, X.-G.; Guan, D.-L.; Zeng, G.; Cao, Z.; Liang, C.; Tang, N.; Zhang, L.; Wen, X.-J.; Zeng, G. Constructing magnetic and high-efficiency AgI/CuFe2O4 photocatalysts for inactivation of Escherichia coli and Staphylococcus aureus under visible light: Inactivation performance and mechanism analysis. Sci. Total Environ. 2019, 668, 730–742. [Google Scholar] [CrossRef]
- Shi, H.; Wang, C.; Zhao, Y.; Liu, E.; Fan, J.; Ji, Z. Highly efficient visible light driven photocatalytic inactivation of E. coli with Ag QDs decorated Z-scheme Bi2S3/SnIn4S8 composite. Appl. Catal. B Environ. 2019, 254, 403–413. [Google Scholar] [CrossRef]
- Wei, F.; Li, J.; Dong, C.; Bi, Y.; Han, X. Plasmonic Ag decorated graphitic carbon nitride sheets with enhanced visible-light response for photocatalytic water disinfection and organic pollutant removal. Chemosphere 2020, 242, 125201. [Google Scholar] [CrossRef]
- Wang, X.; Su, K.; Tan, L.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Lianga, Y.; Li, Z.; Zhu, S.; et al. Rapid and Highly Effective Noninvasive Disinfection by Hybrid Ag/CS@MnO2 Nanosheets Using Near-Infrared Light. ACS Appl. Mater. Interfaces 2019, 11, 15014–15027. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, Z.; Karahan, H.E.; Wang, Y.; Sui, X.; Liu, F.; Chen, Y. Nanocarbon materials in water disinfection: State-of-the-art and future directions. Nanoscale 2019, 11, 9819–9839. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhou, X.; Yan, Y.; Li, Z.; Zheng, T. Enhanced visible-light-driven photocatalytic disinfection using AgBr-modified g-C3N4 composite and its mechanism. Colloids Surf. B: Biointerfaces 2019, 179, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Tan, X.; Hu, Y.; Guo, Q.; Hong, X. Filtration and Electrochemical Disinfection Performance of PAN/PANI/AgNWs-CC Composite Nanofiber Membrane. Environ. Sci. Technol. 2017, 51, 6395–6403. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-T.; Liang, Y.N.; Zhao, J.; Zhang, Y.; Yang, E.-H.; Chen, J.; Lim, T.-T. Ultra-effective integrated technologies for water disinfection with a novel 0D-2D-3D nanostructured rGO-AgNP/Bi2Fe4O9 composite. Appl. Catal. B Environ. 2018, 227, 548–556. [Google Scholar] [CrossRef]
- Hu, Z.T.; Lua, S.K.; Lim, T.T. Cuboid-like Bi2Fe4O9/Ag with Graphene-Wrapping Tribrid Composite with Superior Capability for Environmental Decontamination: Nanoscaled Material Design and Visible-Light-Driven Multifunctional Catalyst. ACS Sustain. Chem. Eng. 2015, 3, 2726–2736. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, D.; Cho, M.; Lee, S.S.; Zhang, F.; Biswas, P.; Fortner, J.D. In Situ Photocatalytic Synthesis of Ag Nanoparticles (nAg) by Crumpled Graphene Oxide Composite Membranes for Filtration and Disinfection Applications. Environ. Sci. Technol. 2016, 50, 2514–2521. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, J.; Wei, F.; Zhao, X.; Su, Y.; Han, X. Ag–ZnO Submicrometer Rod Arrays for High-Efficiency Photocatalytic Degradation of Congo Red and Disinfection. ACS Sustain. Chem. Eng. 2019, 7, 11258–11266. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, Q.; Zhang, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Negishi, N.; Yang, Y. Superior disinfection effect of Escherichia coli by hydrothermal synthesized TiO2-based composite photocatalyst under LED irradiation: Influence of environmental factors and disinfection mechanism. Environ. Pollut. 2019, 247, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.M.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Osmond, M.; Osmond, M.; Oytam, Y.; McCall, M.; Feltis, B.; Mackay-Sim, A.; Wood, S.A.; Cook, A.L. Surface coatings of ZnO nanoparticles mitigate differentially a host of transcriptional, protein and signalling responses in primary human olfactory cells. Part. Fibre Toxicol. 2013, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.K.; Lyon, D.; Alvarez, P.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, X.; Yan, D.; Yin, G.; Liao, X.; Kang, Y.; Yao, Y.; Huang, D.; Hao, B. Toxicological Effect of ZnO Nanoparticles Based on Bacteria. Langmuir 2008, 24, 4140–4144. [Google Scholar] [CrossRef]
- Atmaca, S.; Gul, K.; Cicek, R. The Effect of Zinc On Microbial Growth. Tr. J. Med. Sci. 1998, 28, 595–597. [Google Scholar]
- Lara, H.H.; Garza-Treviño, E.; Turrent, L.I.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.; Lau, G.; Che, C. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 252–262. [Google Scholar]
- Park, S.; Ko, Y.-S.; Lee, S.J.; Lee, C.; Woo, K.; Ko, G. Inactivation of influenza A virus via exposure to silver nanoparticle-decorated silica hybrid composites. Environ. Sci. Pollut. Res. 2018, 25, 27021–27030. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment. Rev. Environ. Health 2017, 32, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.G.C.; Hussain, A.; Marcos, A.; Roque, A. A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol. Adv. 2011, 29, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhang, X.; Wang, Y.; Liu, J.; Pan, B. Mesoporous polyacrylonitrile membrane with ultrahigh loading of well-dispersed Fe2O3 nanoparticles: A powerful phosphate scavenger Enabling inhibition of microbial regrowth in Treated Water. J. Membr. Sci. 2020, 603, 118048. [Google Scholar] [CrossRef]
- Ligneris, E.D.; Dumée, L.F.; Al-Attabi, R.; Castanet, E.; Schutz, J.; Kong, L. Mixed Matrix Poly(Vinyl Alcohol)-Copper Nanofibrous Anti-Microbial Air-Microfilters. Membranes 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of Copper Oxides in Contact Killing of Bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Ding, H.; Han, D.; Han, Y.; Liang, Y.; Liu, X.; Li, Z.; Zhu, S.; Wu, S. Visible light responsive CuS/ protonated g-C3N4 heterostructure for rapid sterilization. J. Hazard. Mater. 2020, 393, 122423. [Google Scholar] [CrossRef]
- Han, D.; Han, Y.; Li, J.; Liu, X.; Yeung, K.W.K.; Zheng, Y.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; et al. Enhanced photocatalytic activity and photothermal effects of cu-doped metal-organic frameworks for rapid treatment of bacteria-infected wounds. Appl. Catal. B: Environ. 2020, 261, 118248. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo)catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, M.; Liu, F.; Dong, Q.; Ma, Z.; Liu, W. Magnetic Fe3O4-deposited flower-like MoS2 nanocomposites for the Fenton-like Escherichia coli disinfection and diclofenac degradation. J. Hazard. Mater. 2020, 385, 121604. [Google Scholar] [CrossRef]
- Wan, J.; Wu, B.; Lo, I.M.C. Development of Fe0/Fe3O4 composites with tunable properties facilitated by Fe2+ for phosphate removal from river water. Chem. Eng. J. 2020, 388, 124242. [Google Scholar] [CrossRef]
- Manohara, H.M.; Aruchamy, K.; Chakraborty, S.; Radha, N.; Nidhi, M.R.; Ghosh, D.; Nataraj, S.K.; Mondal, D. Sustainable Water Purification Using an Engineered Solvothermal Carbon Based Membrane Derived from a Eutectic System. ACS Sustain. Chem. Eng. 2019, 7, 10143–10153. [Google Scholar] [CrossRef]
- Wang, F.-H.; Zheng, T.; Xiong, R.; Wang, P.-P.; Ma, J. CDs@ZIF-8 Modified Thin Film Polyamide Nanocomposite Membrane for Simultaneous Enhancement of Chlorine-Resistance and Disinfection Byproducts Removal in Drinking Water. ACS Appl. Mater. Interfaces 2019, 11, 33033–33042. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; de la Iglesia, Ó.; Fíla, V.; Tellez, C.; Coronas, J.; Figoli, A. Graphene oxide—Filled polyimide membranes in pervaporative separation of azeotropic methanol—MTBE mixtures. Sep. Purif. Technol. 2019, 224, 265–272. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Agrawal, K.V.; Coronas, J. Ultrathin permselective membranes: The latent way for efficient gas separation. RSC Adv. 2020, 10, 12653–12670. [Google Scholar] [CrossRef]
- Oh, Y.; Noga, R.; Shanov, V.; Ryu, H.; Chandra, H.; Yadav, B.; Yadav, J.; Chae, S. Electrically heatable carbon nanotube point-of-use filters for effective separation and in-situ inactivation of Legionella pneumophila. Chem. Eng. J. 2019, 366, 21–26. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Lu, L.; Lin, Z. Reversible switch between bulk MgCO3•3H2O and Mg(OH)2 micro/nanorods induces continuous selective preconcentration of anionic dyes. ACS Appl. Mater. Interfaces 2013, 5, 7698–7703. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, P.; Wang, Z.; Zheng, S.; Zhang, Y. Adsorption study of selenium ions from aqueous solutions using MgO nanosheets synthesized by ultrasonic method. J. Hazard. Mater. 2018, 341, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xia, Y.; Gong, Y.; Li, W.; Li, Z. Efficient natural organic matter removal from water using nano-MgO coupled with microfiltration membrane separation. Sci. Total Environ. 2020, 711, 135120. [Google Scholar] [CrossRef] [PubMed]
- Galiano, F.; Briceno, K.; Marino, T.; De Gisi, S.; Christensen, K.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Valdez, J.; Ahmad, M.Z. High-performance pervaporation chitosan-based membranes: New insights and perspectives. Rev. Chem. Eng. 2020, 1–16. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Castro-Muñoz, R. New Trends in Biopolymer-Based Membranes for Pervaporation. Molecules 2019, 24, 3584. [Google Scholar] [CrossRef] [Green Version]
- Majiya, H.; Chowdhury, K.F.; Stonehouse, N.J.; Millner, P. TMPyP functionalised chitosan membrane for efficient sunlight driven water disinfection. J. Water Process Eng. 2019, 30, 100475. [Google Scholar] [CrossRef]







| Ag Composite | Polymer Matrix | Antibacterial Activity | Performance | Ref. |
|---|---|---|---|---|
| GO–Ag | PVDF | Escherichia coli | Flux: 150–200 L m−2 h−1 | [34] |
| GO–Ag | - | Pseudomonas aeruginosa | Efficiency *: 100% | [36] |
| AgI–CuFe2O4 | - | Staphylococcus aureus Escherichia coli | - | [41] |
| Ag QDs decorated Z-scheme Bi2S3/SnIn4S8 | - | Escherichia coli | Efficiency *: 84% | [42] |
| Ag/g–C3N4 | - | Escherichia coli | Efficiency *: 99% | [43] |
| AgBr/g–C3N4 | Escherichia coli | Efficiency *: 80% | [46] | |
| PAN/PANI/AgNWs–CC | PAN | Escherichia coli Staphylococcus aureus | Efficiency *: 100% | [47] |
| rGO–Ag/Bi2Fe4O9 | - | Escherichia coli Staphylococcus aureus Pseudomonas aeruginosa | Efficiency *: 100% | [48] |
| Ag-crumpled GO nanocomposite | PES | Escherichia coli Bacillus subtilis | Flux: 454 L m−2 h−1 | [51] |
| Ag–ZnO | - | Escherichia coli | Efficiency *: 92% | [52] |
| P/Ag/Ag2O/Ag3PO4/TiO2 | - | Escherichia coli | Efficiency *: 100% | [53] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Muñoz, R. The Role of New Inorganic Materials in Composite Membranes for Water Disinfection. Membranes 2020, 10, 101. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050101
Castro-Muñoz R. The Role of New Inorganic Materials in Composite Membranes for Water Disinfection. Membranes. 2020; 10(5):101. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050101
Chicago/Turabian StyleCastro-Muñoz, Roberto. 2020. "The Role of New Inorganic Materials in Composite Membranes for Water Disinfection" Membranes 10, no. 5: 101. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050101