Aquaporin-Containing Proteopolymersomes in Polyelectrolyte Multilayer Membranes
Abstract
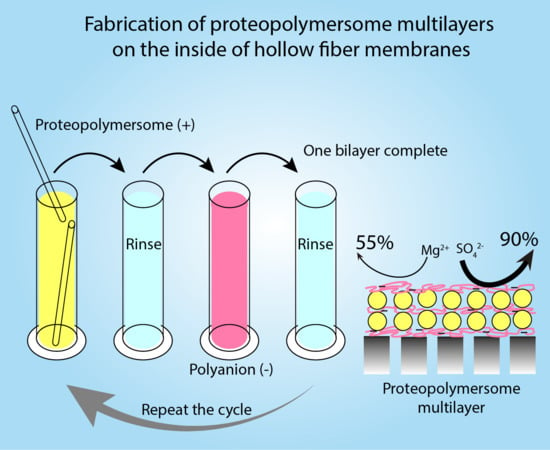
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reflectometry
2.3. Scanning Electron Microscopy
2.4. Transmission Electron Microscopy
2.5. Membrane Performance Experiments
3. Results and Discussion
3.1. Multilayer Characterization
3.2. Membrane Performance
3.3. Literature Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Toutianoush, A.; Jin, W.Q.; Deligoz, H.; Tieke, B. Polyelectrolyte multilayer membranes for desalination of aqueous salt solutions and seawater under reverse osmosis conditions. Appl. Surf. Sci. 2005, 246, 437–443. [Google Scholar] [CrossRef]
- Saren, Q.; Qiu, C.Q.; Tang, C.Y. Synthesis and characterization of novel forward osmosis membranes based on layer-by-layer assembly. Environ. Sci. Technol. 2011, 45, 5201–5208. [Google Scholar] [CrossRef]
- Jin, W.; Toutianoush, A.; Tieke, B. Use of Polyelectrolyte Layer-by-Layer Assemblies as Nanofiltration and Reverse Osmosis Membranes. Langmuir 2003, 19, 2550–2553. [Google Scholar] [CrossRef]
- De Grooth, J. Tale of Two Charges: Zwitterionic Polyelect Rolyte Multilayer Membranes; University of Twente: Enschede, The Netherlands, 2014. [Google Scholar]
- De Grooth, J.; Haakmeester, B.; Wever, C.; Potreck, J.; de Vos, W.M.; Nijmeijer, K. Long term physical and chemical stability of polyelectrolyte multilayer membranes. J. Membr. Sci. 2015, 489, 153–159. [Google Scholar] [CrossRef]
- Shen, Y.X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Chaumont, F.; Barrieu, F.; Wojcik, E.; Chrispeels, M.J.; Jung, R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef]
- Masin, J.; Osickova, A.; Sukova, A.; Fiser, R.; Halada, P.; Bumba, L.; Linhartova, I.; Osicka, R.; Sebo, P. Negatively charged residues of the segment linking the enzyme and cytolysin moieties restrict the membrane-permeabilizing capacity of adenylate cyclase toxin. Sci. Rep. 2016, 6, 29137. [Google Scholar] [CrossRef]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The Critical Need for Increased Selectivity, Not Increased Water Permeability, for Desalination Membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Tang, C.; Wang, Z.; Petrinić, I.; Fane, A.G.; Hélix-Nielsen, C. Biomimetic aquaporin membranes coming of age. Desalination 2015, 368, 89–105. [Google Scholar] [CrossRef]
- Tang, C.Y.; Zhao, Y.; Wang, R.; Helix-Nielsen, C.; Fane, A.G. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Borgnia, M.J.; Kozono, D.; Calamita, G.; Maloney, P.C.; Agre, P. Functional reconstitution and characterization of AqpZ, the E-coli water channel protein. J. Mol. Biol. 1999, 291, 1169–1179. [Google Scholar] [CrossRef]
- Goers, R.; Thoma, J.; Ritzmann, N.; Di Silvestro, A.; Alter, C.; Gunkel-Grabole, G.; Fotiadis, D.; Muller, D.J.; Meier, W. Optimized reconstitution of membrane proteins into synthetic membranes. Commun. Chem. 2018, 1. [Google Scholar] [CrossRef]
- Kowal, J.; Zhang, X.Y.; Dinu, I.A.; Palivan, C.G.; Meier, W. Planar Biomimetic Membranes Based on Amphiphilic Block Copolymers. ACS Macro. Lett. 2014, 3, 59–63. [Google Scholar] [CrossRef]
- Giwa, A.; Hasan, S.W.; Yousuf, A.; Chakraborty, S.; Johnson, D.J.; Hilal, N. Biomimetic membranes: A critical review of recent progress. Desalination 2017, 420, 403–424. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qiu, C.Q.; Li, X.S.; Vararattanavech, A.; Shen, W.M.; Torres, J.; Helix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and RO performance characterization. J. Membr. Sci. 2012, 423, 422–428. [Google Scholar] [CrossRef]
- Gan, H.X.; Zhou, H.; Lee, H.J.; Lin, Q.; Tong, Y.W. Toward a Better Understanding of the Nature-Inspired Aquaporin Biomimetic Membrane. Langmuir 2019, 35, 7285–7293. [Google Scholar] [CrossRef]
- Górecki, R.; Reurink, D.M.; Khan, M.M.; Sanahuja-Embuena, V.; Trzaskuś, K.; Hélix-Nielsen, C. Improved reverse osmosis thin film composite biomimetic membranes by incorporation of polymersomes. J. Membr. Sci. 2020, 593, 117392. [Google Scholar] [CrossRef]
- Sun, G.F.; Chung, T.S.; Chen, N.P.; Lu, X.M.; Zhao, Q.P. Highly permeable aquaporin-embedded biomimetic membranes featuring a magnetic-aided approach. RSC Adv. 2013, 3, 9178–9184. [Google Scholar] [CrossRef]
- Sun, G.F.; Chung, T.S.; Jeyaseelan, K.; Armugam, A. A layer-by-layer self-assembly approach to developing an aquaporin-embedded mixed matrix membrane. RSC Adv. 2013, 3, 473–481. [Google Scholar] [CrossRef]
- Li, X.S.; Chou, S.R.; Wang, R.; Shi, L.; Fang, W.X.; Chaitra, G.; Tang, C.Y.Y.; Torres, J.; Hu, X.; Fane, A.G. Nature gives the best solution for desalination: Aquaporin-based hollow fiber composite membrane with superior performance. J. Membr. Sci. 2015, 494, 68–77. [Google Scholar] [CrossRef]
- Li, X.S.; Loh, C.H.; Wang, R.; Widjajanti, W.; Torres, J. Fabrication of a robust high-performance FO membrane by optimizing substrate structure and incorporating aquaporin into selective layer. J. Membr. Sci. 2017, 525, 257–268. [Google Scholar] [CrossRef]
- Vogel, J.; Groth, J.S.; Nielsen, K.H.; Geschke, O. A Hollow Fiber Module Having Thin Film Composite Aquaporin Modified Membranes WO/2014/108827. International Patent Application No. PCT/IB2014/058096, 7 Jaunary 2014. [Google Scholar]
- Spulber, M.; Trzaskus, K. Self-Assembled Nanostructures and Separation Membranes Comprising Aquaporin Water Channels and Methods of Making and Using Them. WO/2017/137361. International Patent Application No. PCT/EP2017/052567, 6 February 2017. [Google Scholar]
- Shiratori, S.S.; Rubner, M.F. pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Riegler, H.; Essler, F. Polyelectrolytes. 2. Intrinsic or extrinsic charge compensation? Quantitative charge analysis of PAH/PSS multilayers. Langmuir 2002, 18, 6694–6698. [Google Scholar] [CrossRef]
- McAloney, R.A.; Sinyor, M.; Dudnik, V.; Goh, M.C. Atomic Force Microscopy Studies of Salt Effects on Polyelectrolyte Multilayer Film Morphology. Langmuir 2001, 17, 6655–6663. [Google Scholar] [CrossRef]
- Ilyas, S.; Abtahi, S.M.; Akkilic, N.; Roesink, H.D.W.; de Vos, W.M. Weak polyelectrolyte multilayers as tunable separation layers for micro-pollutant removal by hollow fiber nanofiltration membranes. J. Membr. Sci. 2017, 537, 220–228. [Google Scholar] [CrossRef]
- Ouyang, L.; Malaisamy, R.; Bruening, M.L. Multilayer polyelectrolyte films as nanofiltration membranes for separating monovalent and divalent cations. J. Membr. Sci. 2008, 310, 76–84. [Google Scholar] [CrossRef]
- Spulber, M.; Gerstandt, K. Diblock Copolymer Vesicles and Separation Membranes Comprising Aquaporin Water Channels and Methods of Making and Using Them. U.S. Patent No. 16/483,852, 6 February 2018. [Google Scholar]
- Dijt, J.C.; Stuart, M.A.C.; Fleer, G.J. Reflectometry as a Tool for Adsorption Studies. Adv. Colloid Interface Sci. 1994, 50, 79–101. [Google Scholar] [CrossRef]
- Su, C.; Ma, S.M.; Liu, G.X.; Yang, S.G. Dewetting Behavior of Hydrogen Bonded Polymer Complex Film under Hydrothermal Condition. Chin. J. Polym. Sci. 2018, 36, 1036–1042. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Gerberich, W.W. Polymer film rupturing in comparison with leveling and dewetting. Surf. Sci. 2005, 594, 192–202. [Google Scholar] [CrossRef]
- Kolasinska, M.; Krastev, R.; Warszynski, P. Characteristics of polyelectrolyte multilayers: Effect of PEI anchoring layer and posttreatment after deposition. J. Colloid Interface Sci. 2007, 305, 46–56. [Google Scholar] [CrossRef]
- De Grooth, J.; Oborný, R.; Potreck, J.; Nijmeijer, K.; de Vos, W.M. The role of ionic strength and odd–even effects on the properties of polyelectrolyte multilayer nanofiltration membranes. J. Membr. Sci. 2015, 475, 311–319. [Google Scholar] [CrossRef]
- Schönhoff, M.; Ball, V.; Bausch, A.R.; Dejugnat, C.; Delorme, N.; Glinel, K.; Klitzing, R.V.; Steitz, R. Hydration and internal properties of polyelectrolyte multilayers. Colloids Surf. A 2007, 303, 14–29. [Google Scholar] [CrossRef]
- Reurink, D.M.; Haven, J.P.; Achterhuis, I.; Lindhoud, S.; Roesink, H.D.W.; de Vos, W.M. Annealing of Polyelectrolyte Multilayers for Control over Ion Permeation. Adv. Mater. Interfaces 2018, 5, 1800651. [Google Scholar] [CrossRef] [Green Version]
- Ghostine, R.A.; Markarian, M.Z.; Schlenoff, J.B. Asymmetric growth in polyelectrolyte multilayers. J. Am. Chem. Soc. 2013, 135, 7636–7646. [Google Scholar] [CrossRef]
- Sengur-Tasdemir, R.; Sayinli, B.; Urper, G.M.; Tutuncu, H.E.; Gul-Karaguler, N.; Ates-Genceli, E.; Tarabara, V.V.; Koyuncu, I. Hollow fiber nanofiltration membranes with integrated aquaporin Z. New J. Chem. 2018, 42, 17769–17778. [Google Scholar] [CrossRef]
- Li, Y.; Qi, S.; Tian, M.; Widjajanti, W.; Wang, R. Fabrication of aquaporin-based biomimetic membrane for seawater desalination. Desalination 2019, 467, 103–112. [Google Scholar] [CrossRef]
- Qi, S.R.; Fang, W.X.; Siti, W.; Widjajanti, W.; Hu, X.; Wang, R. Polymersomes-based high-performance reverse osmosis membrane for desalination. J. Membr. Sci. 2018, 555, 177–184. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, R.; Wicaksana, F.; Tang, C.Y.; Torres, J.; Fane, A.G. Preparation of high performance nanofiltration (NF) membranes incorporated with aquaporin Z. J. Membr. Sci. 2014, 450, 181–188. [Google Scholar] [CrossRef]
- Xie, W.Y.; He, F.; Wang, B.F.; Chung, T.S.; Jeyaseelan, K.; Armugam, A.; Tong, Y.W. An aquaporin-based vesicle-embedded polymeric membrane for low energy water filtration. J. Mater. Chem. A 2013, 1, 7592–7600. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Tang, C.; Vararattanavech, A.; Zhao, Y.; Torres, J.; Fane, T. Preparation of supported lipid membranes for aquaporin Z incorporation. Colloids Surf. B Biointerfaces 2012, 94, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.D.; Cai, J.; Yu, Z.Y.; Wang, Q.H.; Xu, Z.N.; Wang, Z.N.; Gao, C.J. Fabrication of an aquaporin-based forward osmosis membrane through covalent bonding of a lipid bilayer to a microporous support. J. Mater. Chem. A 2015, 3, 20118–20126. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Wang, X.; Wang, S.; Ding, W.; Gao, C. Layer-by-layer assembly of aquaporin Z-incorporated biomimetic membranes for water purification. Environ. Sci. Technol. 2015, 49, 3761–3768. [Google Scholar] [CrossRef]








| Membrane Type | Incorporation Approach | Permeability (L∙m−2∙h−1∙bar−1) | Salt Concentration | Salt Retention |
|---|---|---|---|---|
| Polyelectrolyte multi-layer (PEM) | ||||
| [PSS/PDADMAC]5PSS | N/A | 5.4 | 5 mM | 9% NaCl, 67% MgCl2 90% Na2SO4, 89% MgSO4 |
| [PSS/PAH]5PSS | N/A | 12.3 | 5 mM | 26% NaCl, 96% MgCl2 80% Na2SO4, 94% MgSO4 |
| [PAA/PAH]5PAA | N/A | 2.6 | 5 mM | 9% NaCl, 57% MgCl2 34% Na2SO4, 60% MgSO4 |
| Proteopolymersome multilayer (PPM) | ||||
| PDADMAC[PSS/PP+]4PSS | LbLa | 3.5 | 5 mM | 8% NaCl, 48% MgCl2 90% Na2SO4, 84% MgSO4 |
| PAH[PSS/PP+]4PSS | LbLa | 7.7 | 5 mM | 11% NaCl, 54% MgCl2 85% Na2SO4, 80% MgSO4 |
| PAH[PAA/PP+]4PAA | LbLa | 0.6 | 5 mM | 8% NaCl, 17% MgCl2 1.0% Na2SO4, 10% MgSO4 |
| Literature values | ||||
| ABM1 [20] | IPb | 4.0 | 10 mM | 97% NaCl |
| ABM2 [25] | IPb | 8.0 | 500 ppm | 97.5% NaCl |
| ABM3 [43] | IPb | 3.2 | 1000 ppm | 69.6% MgSO4, 16.5% NaCl |
| ABM4 [22] | IPb | 6.4 | 500 ppm | 93.5% NaCl |
| ABM5 [44] | IPb | 1.3 | 2000 mg/L | 99.1% NaCl |
| ABM6 [45] | IPb | 2.4 | 2000 ppm | 99.6% NaCl |
| ABM5 [46] | CLc | 36.6 | 100 ppm | 85% MgCl2 |
| ABM6 [47] | Pold | 23 | 200 ppm | 51% MgCl2 |
| ABM7 [24] | LbLa | 6.0 | 200 ppm | 95% MgCl2 |
| ABM8 [48] | SLBe | 4.8 | 1 mM | 20% NaCl |
| ABM9 [49] | SLBe | 6.3 | 2000 ppm | 90% MgCl2 |
| ABM10 [50] | SLBe | 5.5 | 0.5 g/L | 97% MgCl2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reurink, D.M.; Du, F.; Górecki, R.; Roesink, H.D.W.; de Vos, W.M. Aquaporin-Containing Proteopolymersomes in Polyelectrolyte Multilayer Membranes. Membranes 2020, 10, 103. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050103
Reurink DM, Du F, Górecki R, Roesink HDW, de Vos WM. Aquaporin-Containing Proteopolymersomes in Polyelectrolyte Multilayer Membranes. Membranes. 2020; 10(5):103. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050103
Chicago/Turabian StyleReurink, Dennis M., Fei Du, Radosław Górecki, Hendrik D.W. Roesink, and Wiebe M. de Vos. 2020. "Aquaporin-Containing Proteopolymersomes in Polyelectrolyte Multilayer Membranes" Membranes 10, no. 5: 103. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050103