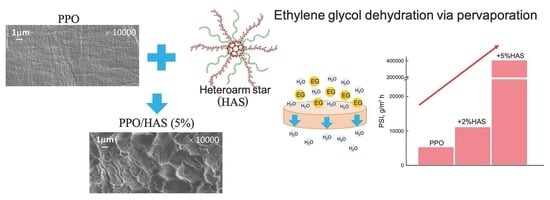
Strongly Selective Polymer Membranes Modified with Heteroarm Stars for the Ethylene Glycol Dehydration by Pervaporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Sorption Study
2.5. Pervaporation
3. Results
3.1. Membrane Characterization
3.2. Transport Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xi, J.; Ding, D.; Shao, Y.; Liu, X.; Lu, G.; Wang, Y. Production of Ethylene Glycol and Its Monoether Derivative from Cellulose. ACS Sustain. Chem. Eng. 2014, 2, 2355–2362. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.M.; Shao, P.; Feng, X.; Anderson, W.A. Separation of Ethylene Glycol−Water Mixtures Using Sulfonated Poly(ether ether ketone) Pervaporation Membranes: Membrane Relaxation and Separation Performance Analysis. Ind. Eng. Chem. Res. 2002, 41, 2957–2965. [Google Scholar] [CrossRef]
- Dye, R.F. Ethylene Glycols Technology. Korean J. Chem. Eng. 2001, 18, 571–579. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Memb. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Otvagina, K.; Penkova, A.; Dmitrenko, M.; Kuzminova, A.; Sazanova, T.; Vorotyntsev, A.; Vorotyntsev, I. Novel Composite Membranes Based on Chitosan Copolymers with Polyacrylonitrile and Polystyrene: Physicochemical Properties and Application for Pervaporation Dehydration of Tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Azimi, H.; Ebneyamini, A.; Tezel, F.; Thibault, J. Separation of Organic Compounds from ABE Model Solutions via Pervaporation Using Activated Carbon/PDMS Mixed Matrix Membranes. Membranes 2018, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Singha, N. Polymeric Nanocomposite Membranes for Next Generation Pervaporation Process: Strategies, Challenges and Future Prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Shahverdi, M.; Baheri, B.; Rezakazemi, M.; Motaee, E.; Mohammadi, T. Pervaporation study of ethylene glycol dehydration through synthesized (PVA-4A)/polypropylene mixed matrix composite membranes. Polym. Eng. Sci. 2013, 53, 1487–1493. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y.M. Pervaporation with chitosan membranes. I. Separation of water from ethylene glycol by a chitosan/polysulfone composite membrane. J. Memb. Sci. 1996, 116, 67–76. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, T.S.; Neo, B.W.; Gruender, M. Processing and engineering of pervaporation dehydration of ethylene glycol via dual-layer polybenzimidazole (PBI)/polyetherimide (PEI) membranes. J. Memb. Sci. 2011, 378, 339–350. [Google Scholar] [CrossRef]
- Ong, Y.T.; Tan, S.H. Synthesis of the novel symmetric buckypaper supported ionic liquid membrane for the dehydration of ethylene glycol by pervaporation. Sep. Purif. Technol. 2015, 143, 135–145. [Google Scholar] [CrossRef]
- Guo, R.; Hu, C.; Pan, F.; Wu, H.; Jiang, Z. PVA-GPTMS/TEOS hybrid pervaporation membrane for dehydration of ethylene glycol aqueous solution. J. Memb. Sci. 2006, 281, 454–462. [Google Scholar] [CrossRef]
- Guo, R.; Ma, X.; Hu, C.; Jiang, Z. Novel PVA-silica nanocomposite membrane for pervaporative dehydration of ethylene glycol aqueous solution. Polymer 2007, 48, 2939–2945. [Google Scholar] [CrossRef]
- Yu, C.; Zhong, C.; Liu, Y.; Gu, X.; Yang, G.; Xing, W.; Xu, N. Pervaporation dehydration of ethylene glycol by NaA zeolite membranes. Chem. Eng. Res. Des. 2012, 90, 1372–1380. [Google Scholar] [CrossRef]
- Hu, S.Y.; Zhang, Y.; Lawless, D.; Feng, X. Composite membranes comprising of polyvinylamine-poly(vinyl alcohol) incorporated with carbon nanotubes for dehydration of ethylene glycol by pervaporation. J. Memb. Sci. 2012, 417–418, 34–44. [Google Scholar] [CrossRef]
- Zhang, W.; Ying, Y.; Ma, J.; Guo, X.; Huang, H.; Liu, D.; Zhong, C. Mixed matrix membranes incorporated with polydopamine-coated metal-organic framework for dehydration of ethylene glycol by pervaporation. J. Memb. Sci. 2017, 527, 8–17. [Google Scholar] [CrossRef]
- Chowdhury, G.; Kruczek, B.; Matsuura, T. Polyphenylene Oxide and Modified Polyphenylene Oxide Membranes: Gas, Vapor, and Liquid Separation; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9781461514831. [Google Scholar]
- Sridhar, S.; Smitha, B.; Ramakrishna, M.; Aminabhavi, T.M. Modified poly(phenylene oxide) membranes for the separation of carbon dioxide from methane. J. Memb. Sci. 2006, 280, 202–209. [Google Scholar] [CrossRef]
- Zhuang, G.L.; Tseng, H.H.; Wey, M.Y. Preparation of PPO-silica mixed matrix membranes by in-situ sol-gel method for H2/CO2 separation. Int. J. Hydrog. Energy 2014, 39, 17178–17190. [Google Scholar] [CrossRef]
- Khayet, M.; Villaluenga, J.P.G.; Godino, M.P.; Mengual, J.I.; Seoane, B.; Khulbe, K.C.; Matsuura, T. Preparation and application of dense poly(phenylene oxide) membranes in pervaporation. J. Colloid Interface Sci. 2004, 278, 410–422. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Penkova, A.V.; Toikka, A.M. Fullerene-containing polyphenylene oxide membranes for pervaporation. Desalination 2006, 200, 400–402. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Lai, C.-L.; Chen, J.-T.; Liu, C.-T.; Huang, S.-H.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R. Hydrophobic composite membranes for separating of water–alcohol mixture by pervaporation at high temperature. Chem. Eng. Sci. 2014, 111, 203–210. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Gladchenko, S.V.; Pen’kova, A.V.; Kuznetsov, V.M.; Toikka, A.M. Synthesis of Fullerene-Polyphenylene Oxide Membranes for Separating Aqueous-Organic Mixtures. Russ. J. Appl. Chem. 2005, 78, 1468–1473. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Penkova, A.V.; Toikka, A.M.; Pientka, Z.; Brozova, L.; Bleha, M. Transport of small molecules through polyphenylene oxide membranes modified by fullerene. Sep. Sci. Technol. 2007, 42, 333–347. [Google Scholar] [CrossRef]
- Polotskaya, G.; Pulyalina, A.; Lebedev, V.; Török, G.; Rudakova, D.; Vinogradova, L. Novel view at hybrid membranes containing star macromolecules using neutron scattering and pervaporation dehydration of acetic acid. Mater. Des. 2020, 186, 108352. [Google Scholar] [CrossRef]
- Pulyalina, A.; Porotnikov, D.; Rudakova, D.; Faykov, I.; Chislova, I.; Rostovtseva, V.; Vinogradova, L.; Toikka, A.; Polotskaya, G. Advanced membranes containing star macromolecules with C 60 core for intensification of propyl acetate production. Chem. Eng. Res. Des. 2018, 135, 197–206. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Krasnopeeva, E.L.; Kalyuzhnaya, L.M.; Saprykina, N.N.; Vinogradova, L.V. Mixed matrix membranes with hybrid star-shaped macromolecules for mono- and dihydric alcohols pervaporation. Sep. Purif. Technol. 2015, 143, 192–200. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Pulyalina, A.Y.; Rostovtseva, V.A.; Toikka, A.M.; Saprykina, N.N.; Vinogradova, L.V. Effect of polystyrene stars with fullerene C 60 cores on pervaporation properties of poly(phenylene oxide) membrane. Polym. Int. 2016, 65, 407–414. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Lebedev, V.T.; Pulyalina, A.Y.; Vinogradova, L.V. Structure and transport properties of pervaporation membranes based on polyphenylene oxide and heteroarm star polymers. Pet. Chem. 2016, 56, 920–930. [Google Scholar] [CrossRef]
- Pulyalina, A.Y.; Tataurov, M.V.; Larkina, A.A.; Faykov, I.I.; Rostovtseva, V.A.; Vinogradova, L.V.; Polotskaya, G.A. Pervaporation Desulfurization of a Thiophene/n-Octane Mixture Using PPO Membranes Modified with Hybrid Star-Shaped Macromolecules. Membr. Membr. Technol. 2019, 1, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.R.; Newman, S. Polymer Blends; Media Wiley Com; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Vinogradova, L.V.; Lavrenko, P.N.; Amsharov, K.Y.; Zgonnik, V.N. New Star-Shaped Fullerene-Core Hybrid Polymers Based on Styrene and tert-Butyl Methacrylate. Polym. Sci. Ser. A 2002, 44, 447–453. [Google Scholar]
- Belmares, M.; Blanco, M.; Goddard, W.A.; Ross, R.B.; Caldwell, G.; Chou, S.H.; Pham, J.; Olofson, P.M.; Thomas, C. Hildebrand and hansen solubility parameters from molecular dynamics with applications to electronic nose polymer sensors. J. Comput. Chem. 2004, 25, 1814–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijmans, J.G.; Baker, R.W. A simple predictive treatment of the permeation process in pervaporation. J. Memb. Sci. 1993, 79, 101–113. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Memb. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Lebedev, V.T.; Torok, G.; Vinogradova, L.V. Structure and Supramolecular Structures of Star-Shaped Fullerene-Containing Heteroarm Polymers in Deuterotoluene. Polym. Sci. Ser. A 2011, 53, 12–23. [Google Scholar] [CrossRef]
- Paul, D.R.; Newman, S. Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1, ISBN 9780471248255. [Google Scholar]
- Ilinitch, O.M.; Fenelonov, V.B.; Lapkin, A.A.; Okkel, L.G.; Terskikh, V.V.; Zamaraev, K.I. Intrinsic microporosity and gas transport in polyphenylene oxide polymers. Microporous Mesoporous Mater. 1999, 31, 97–110. [Google Scholar] [CrossRef]
- Pulyalina, A.; Rostovtseva, V.; Polotskaya, G.; Vinogradova, L.; Zoolshoev, Z.; Simonova, M.; Hairullin, A.; Toikka, A.; Pientka, Z. Hybrid macromolecular stars incorporated poly(phenylene oxide) membranes: Organization, physical, and gas separation properties. Polymer 2019, 172, 355–364. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Penkova, A.; Polotskaya, G.; Toikka, A. Pervaporation composite membranes for ethyl acetate production. Chem. Eng. Process. Process. Intensif. 2015, 87, 81–87. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Agranova, S.A.; Gazdina, N.V.; Kuznetsov, Y.P.; Nesterov, V.V. Effect of molecular weight parameters on gas transport properties of poly(2,6-dimethyl-1,4-phenylene oxide). J. Appl. Polym. Sci. 1996, 62, 2215–2218. [Google Scholar] [CrossRef]
- Polotsky, A.E.; Polotskaya, G.A. Study on top layer structure of composite membranes. J. Memb. Sci. 1998, 140, 97–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhim, J.W.; Feng, X. Improving the stability of layer-by-layer self-assembled membranes for dehydration of alcohol and diol. J. Memb. Sci. 2013, 444, 22–31. [Google Scholar] [CrossRef]
- Hyder, M.N.; Huang, R.Y.M.; Chen, P. Composite poly(vinyl alcohol)-poly(sulfone) membranes crosslinked by trimesoyl chloride: Characterization and dehydration of ethylene glycol-water mixtures. J. Memb. Sci. 2009, 326, 363–371. [Google Scholar] [CrossRef]
- Wu, J.K.; Ye, C.C.; Zhang, W.H.; Wang, N.X.; Lee, K.R.; An, Q.F. Construction of well-arranged graphene oxide/polyelectrolyte complex nanoparticles membranes for pervaporation ethylene glycol dehydration. J. Memb. Sci. 2019, 577, 104–112. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lee, Y.M. Pervaporation of ethylene glycol-water mixtures. I. Pervaporation performance of surface crosslinked chitosan membranes. J. Memb. Sci. 1999, 153, 155–162. [Google Scholar]
- Wu, X.M.; Guo, H.; Soyekwo, F.; Zhang, Q.G.; Lin, C.X.; Liu, Q.L.; Zhu, A.M. Pervaporation Purification of Ethylene Glycol Using the Highly Permeable PIM-1 Membrane. J. Chem. Eng. Data 2016, 61, 579–586. [Google Scholar] [CrossRef]







| Membrane | Density, g cm−3 | Water Contact Angle, ° | EG Contact Angle, ° | Critical Surface Tension, mJ/m2 | ||
|---|---|---|---|---|---|---|
| σps | σds | σs | ||||
| PPO | 1.057 | 89 | 63 | 3.9 | 24.0 | 27.9 |
| PPO/HAS (2%) | 1.060 | 91 | 65 | 3.2 | 24.1 | 27.3 |
| PPO/HAS (5%) | 1.064 | 93 | 68 | 2.9 | 22.6 | 25.5 |
| Liquid | Mol. Weight | Density, g/cm3 | Mol. Volume, cm3/mol | Dynamic Viscosity, mPa·s | Solubility Parameter δ, (J/cm3)1/2 |
|---|---|---|---|---|---|
| Water | 18.0 | 0.997 | 18.0 | 1.0 | 49.6 |
| Ethylene glycol | 62.1 | 1.114 | 55.6 | 26.0 | 32.9 |
| Membrane | Equilibrium Sorption Degree, g Liquid/100 g Polymer | |
|---|---|---|
| Water | Ethylene Glycol | |
| PPO | 0 | 4.2 |
| PPO/HAS (2%) | 1.2 | 4.9 |
| PPO/HAS (5%) | 2.9 | 5.4 |
| Membrane | T, °C | PSI, kg/m2 h | Separation Factor βwater/EG | Flux, g/m2 h | Ref. |
|---|---|---|---|---|---|
| CS/PS | 35 | 31 | 104 | 300 | [10] |
| PBI/PEI | 60 | 203 | 1763 | 115 | [11] |
| PVA | 30 | 208 | 802 | 26 | [12] |
| BP-SILM-70 | 30 | 103 | 1014 | 102 | [12] |
| SO3H-MIL-101-Cr@PD-PVA | 70 | 1566 | 2900 | 540 | [17] |
| PA-polyelectrolytes | 22 | 5 | 415 | 12 | [45] |
| PVA/PS | 60 | 355 | 987 | 360 | [46] |
| PEC NPM | 60 | 681 | 470 | 1453 | [47] |
| GFT1001(PVA/PAN) | 75 | 249 | 1116 | 224 | [48] |
| DEG167(PVA/PAN) | 75 | 495 | 991 | 500 | [48] |
| PIM-1 | 30 | 17 | 92 | 186 | [49] |
| PPO/HAS (5 wt%) | 50 | 231 | 11,240 | 20.6 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostovtseva, V.; Pulyalina, A.; Rudakova, D.; Vinogradova, L.; Polotskaya, G. Strongly Selective Polymer Membranes Modified with Heteroarm Stars for the Ethylene Glycol Dehydration by Pervaporation. Membranes 2020, 10, 86. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050086
Rostovtseva V, Pulyalina A, Rudakova D, Vinogradova L, Polotskaya G. Strongly Selective Polymer Membranes Modified with Heteroarm Stars for the Ethylene Glycol Dehydration by Pervaporation. Membranes. 2020; 10(5):86. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050086
Chicago/Turabian StyleRostovtseva, Valeriia, Alexandra Pulyalina, Daria Rudakova, Ludmila Vinogradova, and Galina Polotskaya. 2020. "Strongly Selective Polymer Membranes Modified with Heteroarm Stars for the Ethylene Glycol Dehydration by Pervaporation" Membranes 10, no. 5: 86. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10050086