WO3/Buckypaper Membranes for Advanced Oxidation Processes
Abstract
:1. Introduction
- The need of high surface area, which can be overcome by using nanometer sized materials; and
- The semiconductor recovery after their use, which can be solved, for example, by nano-semiconductors with a magnetic core, by chemically cross-linked semiconductor nanoparticles onto polymer or ceramic membranes and, more recently, by vapor deposition of thin semiconductor films onto suitable substrates [11].
2. Materials and Methods
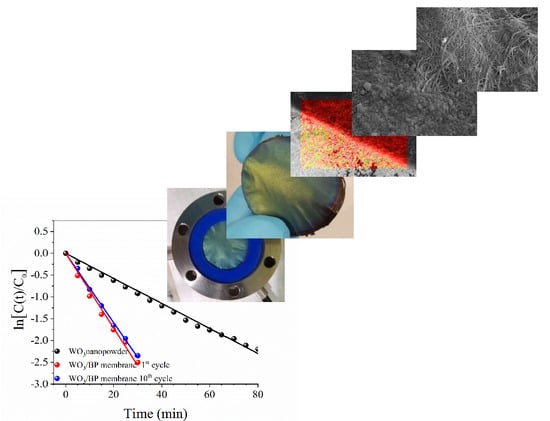
2.1. Preparation of BP Membranes
2.2. Deposition of WO3 onto BP Membranes
2.3. Characterization of BP and WO3/BP Membranes
2.4. Photodegradation Experiments
3. Results and Discussion
- -
- The increase of the number of photons available per BM molecule;
- -
- the higher amount of available catalytically active sites per BM molecule; and
- -
- an easier penetration of photons through the less concentrated solutions.
4. Conclusions
- The possibility to make heterogeneous photocatalytical processes with an easier catalyst recovery and reuse;
- their application in continuous flow plants;
- a simpler and cleaner synthetic approach. Chemical vapor deposition processes do not require long and expensive purification procedures, which are necessary in other chemical syntheses, such as solvo-thermal processes. In addition, CVD allows the catalyst amount saving, avoiding its dispersion in the substrate bulk;
- a higher photocatalytical efficiency, due to the facilitated electron-transfer between carbon nanostrucutres and catalyst nanoparticles, a reduced recombination between electrons and holes [51], and the presence of catalyst nanoparticles with small size just only on the top surface of substrates rather than in the polymer bulk (where they cannot play any catalytic action);
- the possibility to change the photocatalyst crystal structure in a more photoactive one by thermal annealing processes at temperatures higher than the melting point of commonly used polymer substrate. PTFE, polytetrafluoroethylene, which has one of the highest melting points, melts at 327 °C, a temperature lower than the WO3 amorphous-monoclinic phase transition temperature. On the contrary, BP membranes result thermally stable up to 400 °C;
- BPs have both light weight and strong mechanical resistance, and, consequently, are easy to handle. In addition, BPs are resistant to all organic solvents and acid and base solutions, while porous polymer membranes can be damaged; and
- a green chemisty approach with an almost zero environmental footprint, as the BP preparation is based on rather simple and clean experimental set-ups, which allow the recovery and reuse of solvents, CNT processing waste, end of life BPs and photocatalysts for the preparation of new catalyst/BP membranes.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Photo-Catalysis Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 1989. [Google Scholar]
- Araña, J.; Herrera Melián, J.A.; Doña Rodríguez, J.M.; González Díaz, O.; Viera, A.; Pérez Peña, J.; Marrero Sosa, P.M.; Jiménez, V.E. TiO2-photocatalysis as a tertiary treatment of naturally treated wastewater. Catal. Today 2002, 76, 279–289. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Leggio, A.; Le Pera, A.; Liguori, A.; Siciliano, C. Optically pure N-hydroxy-O-triisopropylsilyl-α-L-amino acid methyl esters from AlCl3-assisted ring opening of chiral oxaziridines by nitrogen containing nucleophiles. J. Org. Chem. 2005, 70, 10494–10501. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- De Filpo, G.; Palermo, A.M.; Tolmino, R.; Formoso, P.; Nicoletta, F.P. Gellan gum hybrid hydrogels for the cleaning of paper artworks contaminated with Aspergillus versicolor. Cellulose 2016, 23, 3265–3279. [Google Scholar] [CrossRef]
- Huang, Z.; Maness, P.-C.; Blake, D.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A 2000, 130, 163–170. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Munno, R.; Molinaro, L.; Formoso, P.; Nicoletta, F.P. Gellan gum/titanium dioxide nanoparticle hybrid hydrogels for the cleaning and disinfection of parchment. Int. Biodeterior. Biodegrad. 2015, 103, 51–58. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photo-catalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A. Photocatalytic degradation for environmental applications: A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- De Filpo, G.; Pantuso, E.; Armentano, K.; Formoso, P.; Di Profio, G.; Poerio, T.; Fontananova, E.; Meringolo, C.; Mashin, A.I.; Nicoletta, F.P. Chemical vapor deposition of photocatalyst nanoparticles on PVDF membranes for advanced oxidation processes. Membranes 2018, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, D. Functionalized membranes and environmental applications. Clean Technol. Environ. Policy 2007, 9, 81–83. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Human pharmaceuticals in the aquatic environment: A review. Environ. Technol. 2001, 22, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Ziylan, A.; Ince, N.H. The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: Treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Formoso, P.; Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Electro-conductive membranes for permeation enhancement and fouling mitigation: A short review. Membranes 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoletta, F.P.; Cupelli, D.; Formoso, P.; De Filpo, G.; Colella, V.; Gugliuzza, A. Light responsive polymer membranes: A review. Membranes 2012, 2, 134–197. [Google Scholar] [CrossRef] [Green Version]
- Salehi, S.M.; Di Profio, G.; Fontananova, E.; Nicoletta, F.P.; Curcio, E.; De Filpo, G. Membrane distillation by novel hydrogel composite membranes. J. Membr. Sci. 2016, 504, 220–229. [Google Scholar] [CrossRef]
- Salminen, J.; Garbarino, E.; Orveillon, G.; Saveyn, H.; Mateos Aquilino, V.; Llorens González, T.; García Polonio, F.; Horckmans, L.; D’Hugues, P.; Balomenos, E.; et al. Recovery of Critical and Other Raw Materials from Mining Waste and Landfills: State of Play on Existing Practices; EUR 29744 EN; Blengini, G.A., Mathieux, F., Mancini, L., Nyberg, M., Viegas, H.M., Eds.; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-08568-3. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Ngo, H.H.; Kim, J.H. Chemical coupling of photocatalysis with flocculation and adsorption in the removal of organic matter. Water Res. 2005, 39, 2549–2558. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W. Hybridization of photocatalysis and membrane distillation for purification of wastewater. Catal. Today 2006, 118, 181–188. [Google Scholar] [CrossRef]
- Ho, D.P.; Vigneswaran, S.; Ngo, H.H. Integration of photocatalysis and microfiltration in removing effluent organic matter from treated sewage effluent. Sep. Sci. Technol. 2010, 45, 155–162. [Google Scholar] [CrossRef]
- Mendez-Arriaga, F.; Esplugas, S.; Gimenez, J. Photocatalytic degradation of nonsteroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation. Water Res. 2008, 42, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Muramatsu, H.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Fabrication of high-purity, double-walled carbon nanotube buckypaper. Chem. Vap. Depos. 2006, 12, 327–330. [Google Scholar] [CrossRef]
- Endo, M.; Muramatsu, H.; Hayashi, T.; Kim, Y.A.; Terrones, M.; Dresselhaus, M.S. Nanotechnology: ‘buckypaper’ from coaxial nanotubes. Nature 2005, 433, 476. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.-O.; Ralph, S.F. Carbon nanotube membranes: Synthesis, properties, and future filtration applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Frizzell, C.J.; Coutinho, D.H.; Balkus, K.J.; Minett, A.I.; Blau, W.J.; Coleman, J.N. Reinforcement of macroscopic carbon nanotube structures by polymer intercalation: The role of polymer molecular weight and chain conformation. Phys. Rev. B 2005, 72, 245420. [Google Scholar] [CrossRef] [Green Version]
- Vohrer, U.; Kolaric, I.; Haque, M.H.; Roth, S.; Detlaff-Weglikowska, U. Carbon nanotube sheets for the use as artificial muscles. Carbon 2004, 42, 1159–1164. [Google Scholar] [CrossRef]
- Coleman, J.N.; Blau, W.J.; Dalton, A.B.; Muñoz, E.; Collins, S.; Kim, B.G.; Razal, J.; Selvidge, M.; Vieiro, G.; Baughman, R.H.; et al. Improving the mechanical properties of single-walled carbon nanotube sheets by intercalation of polymeric adhesives. Appl. Phys. Lett. 2003, 82, 1682–1684. [Google Scholar] [CrossRef]
- Boge, J.; Sweetman, L.J.; Ralph, S.F. The effect of preparation conditions and biopolymer dispersants on the properties of SWNT buckypapers. J. Mater. Chem. A 2009, 19, 9131–9140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Li, J.M.; Liu, G.G.; Chen, X.L.; Jiang, K. Photodegradation of diclofenac in aqueous solution by simulated sunlight irradiation: Kinetics, thermodynamics and pathways. Water. Sci. Technol. 2017, 75, 2163–2170. [Google Scholar] [CrossRef]
- Russo, F.; Ursino, C.; Avruscio, E.; Desiderio, G.; Perrone, A.; Santoro, S.; Galiano, F.; Figoli, A. Innovative Poly (Vinylidene Fluoride) (PVDF) electrospun nanofiber membrane preparation using DMSO as a low toxicity solvent. Membranes 2020, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, B.; Guan, J.; Mirjalili, V.; Hubert, P.; Simard, B.; Johnston, A. Correlation between Young’s modulus and impregnation quality of epoxy-impregnated SWCNT buckypaper. Compos. Part A 2010, 41, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Whitby, R.L.D.; Fukuda, T.; Maekawa, T.; James, S.L.; Mikhalovsk, S.V. Geometric control and tuneable pore size distribution of buckypaper and buckydiscs. Carbon 2008, 46, 946–956. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.-D.; Oktiani, R.; Ragadhita, R.; Sukmafitri, A.; Zaen, R. Amorphous content on the photocatalytic performance of micrometer-sized tungsten. Arab. J. Chem. 2020, 13, 2912–2924. [Google Scholar] [CrossRef]
- de Wijs, G.A.; de Groot, R.A. Amorphous WO3: A first principles approach. Electrochim. Acta 2001, 46, 1989–1993. [Google Scholar] [CrossRef]
- Tagtstrom, P.; Jansson, U. Chemical vapour deposition of epitaxial WO3 films. Thin Solid Film. 1999, 352, 107–113. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and Raman study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide tydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Díaz-Reyes, J.; Castillo-Ojeda, R.; Galván-Arellano, M.; Zaca-Moran, O. Characterization of WO3 thin films grown on silicon by HFMOD. Adv. Condens. Matter Phys. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, M.; White, T. Degradation of methylene blue by three-dimensionally ordered macroporous titania. Environ. Sci. Technol. 2007, 41, 4405–4409. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Ku, Y. Photocatalytic degradation of Reactive Red 22 in aqueous solution by UV-LED radiation. Water Res. 2006, 40, 2249–2258. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Meringolo, C.; Poerio, T.; Scarpelli, F.; Godbert, N.; Di Profio, G.; Fontananova, E. Multistimuli activation of TiO2/α-alumina membranes for degradation of methylene blue. Ind. Eng. Chem. Res. 2017, 56, 11049–11057. [Google Scholar] [CrossRef]
- Gómez-Solís, C.; Juárez-Ramírez, I.; Moctezuma, E.; Torres-Martínez, L.M. Photodegradation of indigo carmine and methylene blue dyes in aqueous solution by SiC–TiO2 catalysts prepared by sol–gel. J. Hazard. Mater. 2012, 217–218, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Devarahosahalli Veeranna, K.; Theeta Lakshamaiah, M.; Thimmasandra Naraya, R. Photocatalytic degradation of indigo carmine dye using calcium oxide. Int. J. Photochem. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, M.J.; Silva, C.G.; Marques, R.R.N.; Silva, A.M.T.; Faria, J.L. Carbon nanotube–TiO2 thin films for photocatalytic applications. Catal. Today 2011, 161, 91–96. [Google Scholar] [CrossRef]
- Singh, J.; Chang, Y.-Y.; Koduru, J.R.; Yang, J.-K. Potential degradation of methylene blue (MB) by nano-metallic particles: A kinetic study and possible mechanism of MB degradation. Environ. Eng. Res. 2018, 23, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, M.J.; Marques, R.R.N.; Tavares, P.B.; Faria, J.L.; Silva, A.M.T.; Silva, C.G. Tailoring the properties of immobilized titanium dioxide/carbon nanotube composites for photocatalytic water treatment. J. Environ. Chem. Eng. 2013, 1, 945–953. [Google Scholar] [CrossRef]
- Zhou, X.; Shia, T.; Zhou, H. Hydrothermal preparation of ZnO-reduced graphene oxide hybrid with high performance in photocatalytic degradation. Appl. Surf. Sci. 2012, 258, 6204–6211. [Google Scholar] [CrossRef]
- Tian, L.; Ye, L.; Deng, K.; Zan, L. TiO2/carbon nanotube hybrid nanostructures: Solvothermal synthesis and their visible light photocatalytic activity. J. Solid State Chem. 2011, 184, 1465–1471. [Google Scholar] [CrossRef]
- Bojarska, M.; Nowak, B.; Skowroński, J.; Piątkiewicz, W.; Gradoń, L. Growth of ZnO nanowires on polypropylene membrane surface—Characterization and reactivity. Appl. Surf. Sci. 2017, 391, 457–467. [Google Scholar] [CrossRef]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids 2018, 116, 137–147. [Google Scholar] [CrossRef]










| Property | Value |
|---|---|
| Thickness | 45 ± 2 μm |
| Diameter | 37.0 ± 0.1 mm |
| Density | 0.60 ± 0.03 g·cm−3 |
| Porosity | 70 ± 5 % |
| Electrical Conductivity | 83 ± 4 S cm−1 |
| Tensile strength | 11.8 ± 2.2 MPa |
| Fracture Strain | 2.6 ± 0.1% |
| Young’s Modulus | 0.9 ± 0.1 GPa |
| Water Flow Rate | 12,500 ± 100 L m−2·h−1·bar−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Filpo, G.; Pantuso, E.; Mashin, A.I.; Baratta, M.; Nicoletta, F.P. WO3/Buckypaper Membranes for Advanced Oxidation Processes. Membranes 2020, 10, 157. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10070157
De Filpo G, Pantuso E, Mashin AI, Baratta M, Nicoletta FP. WO3/Buckypaper Membranes for Advanced Oxidation Processes. Membranes. 2020; 10(7):157. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10070157
Chicago/Turabian StyleDe Filpo, Giovanni, Elvira Pantuso, Aleksander I. Mashin, Mariafrancesca Baratta, and Fiore Pasquale Nicoletta. 2020. "WO3/Buckypaper Membranes for Advanced Oxidation Processes" Membranes 10, no. 7: 157. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10070157