Antifungal Properties of Nerolidol-Containing Liposomes in Association with Fluconazole
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Liposomes
2.2. Physicochemical Characterization of Liposomes
2.3. Analysis of Antifungal Activity
2.3.1. Strains and Culture Media
2.3.2. Drugs
2.3.3. Analysis of the Cell Viability and Determination of Inhibitory Concentration 50%-IC50
2.3.4. Determination of the Minimum Fungicidal Concentration (MFC)
2.3.5. Evaluation of the Antifungal Enhancing Activity in Association with Fluconazole
2.3.6. Analysis of Candida Morphological Changes
2.4. Statistical Analysis
3. Results
3.1. In Vitro Antifungal Activity of Nerolidol Alone and Incorporated into Liposomes
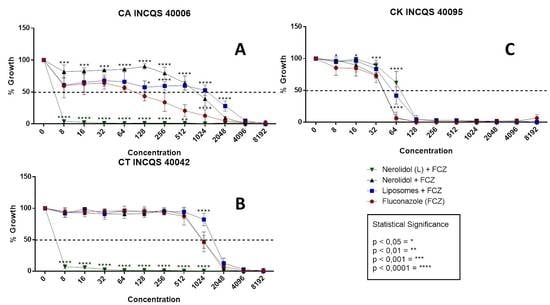
3.2. Antifungal-Enhancing Activity of Liposomal Nerolidol in Association with Fluconazole
3.3. Effects of the Treatments on Fungal Morphology
3.4. Physicochemical Characterization of Liposomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arendrup, M.C. Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care. 2010, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Shigemura, K.; Yoshida, H.; Fujisawa, M.; Arakawa, S. Candida urinary tract infection and Candida species susceptibilities to antifungal agents. J. Antibiot. 2013, 66, 651–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nucci, M.; Colombo, A.L. Candidemia due to Candida tropicalis: Clinical, epidemiologic, and microbiologic characteristics of 188 episodes occurring in tertiary care hospitals. Diagn. Microbiol. Infect. Dis. 2007, 58, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Chim, C.; Ho, P.L.; Cheng, C.; Yuen, K.-Y.; Lie, A.; Au, W.; Liang, R.; Kwong, Y.-L. Candida tropicalis fungaemia in adult patients with haematological malignancies: Clinical features and risk factors. J. Hosp. Infect. 2002, 50, 316–319. [Google Scholar] [CrossRef]
- Barac, A.; Cevik, M.; Colovic, N.; Lekovic, D.; Stevanovic, G.; Micic, J.; Rubino, S. Investigation of a healthcare-associated Candida tropicalis candidiasis cluster in a haematology unit and a systematic review of nosocomial outbreaks. Mycoses 2020, 63, 326–333. [Google Scholar] [CrossRef]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The changing epidemiology of invasive fungal infections. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; pp. 17–65. [Google Scholar]
- Benitez, L.L.; Carver, P.L. Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef]
- Martins, M.; Henriques, M.; Azeredo, J.; Rocha, S.M.; Coimbra, M.A.; Oliveira, R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot Cell 2007, 6, 2429–2436. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.M.; Barreto, A.L.S.; Batista, E.M.; Curvelo, J.A.D.R.; Velozo, L.S.M.; Moreira, D.D.L.; Guimarães, E.F.; Soares, R.M.A.; Kaplan, M.A.C. Chemistry and biological activity of essential oils from Piper claussenianum (Piperaceae). Nat. Prod. Commun. 2010, 5, 1837–1840. [Google Scholar]
- Han, T.L.; Tumanov, S.; Cannon, R.D.; Villas-Boas, S.G. Metabolic response of Candida albicans to phenylethyl alcohol under hyphae-inducing conditions. PLoS ONE 2013, 8, e71364. [Google Scholar] [CrossRef]
- Alonso, L.; Fernandes, K.S.; Mendanha, S.A.; Gonçalves, P.J.; Gomes, R.S.; Dorta, M.L.; Alonso, A.; Neto, S.A.M. In vitro antileishmanial and cytotoxic activities of nerolidol are associated with changes in plasma membrane dynamics. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 1861, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Brehm-Stecher, B.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to Antibiotics by the Sesquiterpenoids Nerolidol, Farnesol, Bisabolol, and Apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Gwak, K.; Yang, I.; Kim, K.; Jeung, E.; Chang, J.; Choi, I. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoter 2009, 80, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Johann, S.; Oliveira, F.B.; Siqueira, E.P.; Cisalpino, P.S.; Rosa, C.A.; Alves, T.M.A.; Zani, C.L.; Cota, B.B. Activity of compounds isolated fromBaccharis dracunculifolia D.C. (Asteraceae)against Paracoccidioides brasiliensis. Med. Mycol. 2012, 50, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.M.; Lin, J.Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Triana, J.; Eiroa, J.L.; Morales, M.; Perez, F.J.; Brouard, I.; Marrero, M.T.; Estévez, S.; Quintana, J.; Estévez, F.; Castillo, Q.A.; et al. A chemotaxonomic study of endemic species of genus Tanacetum from the Canary Islands. Phytochemistry 2013, 92, 87–104. [Google Scholar] [CrossRef]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [Green Version]
- McGinty, D.; Letizia, C.; Api, A. Addendum to Fragrance material review on Nerolidol (isomer unspecified). Food Chem. Toxicol. 2010, 48, S43–S45. [Google Scholar] [CrossRef]
- Azzi, J.; Auezova, L.; Danjou, P.-E.; Fourmentin, S.; Greige-Gerges, H. First evaluation of drug-in-cyclodextrin-in-liposomes as an encapsulating system for nerolidol. Food Chem. 2018, 255, 399–404. [Google Scholar] [CrossRef]
- Bucak, S.; Çağdaş, M. Liposomes as Potential Drug Carrier Systems for Drug Delivery. In Application of Nanotechnology in Drug Delivery; InTech: Rijeka, Croatia, 2014; pp. 1–100. [Google Scholar]
- Škorpilová, L.; Rimpelová, S.; Jurášek, M.; Buděšínský, M.; Lokajová, J.; Effenberg, R.; Slepička, P.; Ruml, T.; Kmonícková, E.; Drasar, P.B.; et al. BODIPY-based fluorescent liposomes with sesquiterpene lactone trilobolide. Beilstein J. Org. Chem. 2017, 13, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Barros, N.B.; Migliaccio, V.; Facundo, V.A.; Ciancaglini, P.; Stabeli, R.G.; Nicolete, R.; Silva-Jardim, I. Liposomal-lupane system as alternative chemotherapy against cutaneous leishmaniasis: Macrophage as target cell. Exp. Parasitol. 2013, 135, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Committee for Clinical Laboratory Standards (NCCLS). Performance Standards for Antimicrobial Susceptibility Testing. Document M100–S12; National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 2002. [Google Scholar]
- Stoppa, M.A.; Casemiro, L.A.; Vinholis, A.H.C.; Cunha, W.R.; e Silva, M.L.A.; Martins, C.H.G.; Furtado, N.A.J.C. Estudo comparativo entre as metodologias preconizadas pelo CLSI e pelo EUCAST para avaliação da atividade antifúngica. Química Nova 2009, 32, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Javadpour, M.M.; Juban, M.M.; Lo, W.-C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De Novo Antimicrobial Peptides with Low Mammalian Cell Toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.F.B.M.; Carneiro, J.N.P.; Machado, A.J.T.; Dos Santos, A.T.L.; Sales, D.L.; Lima, L.F.; Figueredo, F.G.; Coutinho, H.D.M. Psidium guajava L., from ethnobiology to scientific evaluation: Elucidating bioactivity against pathogenic microorganisms. J. Ethnopharmacol. 2016, 194, 1140–1152. [Google Scholar] [CrossRef]
- Ernst, E.J.; Klepser, E.M.; Ernst, E.M.l; Messer, A.S.; Pfaller, A.M. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 1999, 33, 75–80. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; Costa, J.G.M.; Siqueira-Júnior, J.P.; Lima, E.O. In vitro anti-staphylococcal activity of Hyptis martiusii Benth against methicillin-resistant Staphylococcus aureus: MRSA strains. Rev. Bras. Farm. 2008, 18, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Sidrin, J.J.C.; Rocha, M.F.G. Micologia Médica à Luz de Autores Contemporâneos; Guanabara Koogan: Rio de Janeiro, Brazil, 2010; p. 388. [Google Scholar]
- Mendes, J.M. Investigação da atividade antifúngica do óleo essencial de Eugenia caryophyllata Thunb. sobre cepas de Candida tropicalis. In Dissertação de Mestrado em Produtos Naturais e Sintéticos Bioativos; Universidade Federal da Paraíba–UFPB: João Pesso, Paraíba, Brazil, 2011. [Google Scholar]
- Carneiro, J.N.P.; Da Cruz, R.P.; Da Silva, J.C.P.; Rocha, J.E.; De Freitas, T.S.; Sales, D.L.; Bezerra, C.F.; Almeida, W.D.O.; Da Costa, J.G.M.; Da Silva, L.E.; et al. Piper diospyrifolium Kunth.: Chemical analysis and antimicrobial (intrinsic and combined) activities. Microb. Pathog. 2019, 136, 103700. [Google Scholar] [CrossRef]
- Hollis, L.; Jones, R.S. US Environmental Protection Agency Office of Pesticide Programs. Biopestic. Pollut. Prev. Div. Farnesol. Nerolidol. 2009, 1, 24. [Google Scholar]
- El-Kattan, A.F.; Asbill, C.S.; Kim, N.; Michniak, B.B. The effects of terpene enhancers on the percutaneous permeation of drugs with diferente lipophilicities. Int. J. Pharm. 2001, 215, 229–240. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Sharabiani, K.; Rashidi, M.-R.; Ghafourian, T. The effect of terpene concentrations on the skin penetration of diclofenac sodium. Int. J. Pharm. 2007, 335, 97–105. [Google Scholar] [CrossRef]
- Alviano, C.S.; Mendonca-Filho, R.R.; Bizzo, H.R.; Souto-Padrón, T.; Rodrigues, M.L.; Bolognese, A.M.; Souza, M.M.G. Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol. Immunol. 2005, 20, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res. Microbiol. 2010, 161, 816–823. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Lai, W.-L.; Chuang, K.-C.; Lee, M.-H.; Tsai, Y.-C. The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol. 2013, 51, 473–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, R.; Wang, C.-Z.; Kong, Z.-W. Antibacterial/Antifungal Activity and Synergistic Interactions between Polyprenols and Other Lipids Isolated from Ginkgo biloba L. Leaves. Molecules 2013, 18, 2166–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curvelo, J.A.R.; Marques, A.M.; Barreto, A.L.S.; Romanos, M.T.V.; Portela, M.B.; Kaplan, M.A.C.; & Soares, R.M.A. A novel nerolidol-rich essential oil from Piper laussenianum modulates Candida albicans biofilm. J. Med. Microbiol. 2014, 63, 697–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef] [PubMed]
- Pontin, M.; Bottini, R.; Burba, J.L.; Piccoli, P. Allium sativum produces terpenes with fungistatic properties in response to infection with Sclerotium cepivorum. Phytochemistry 2015, 115, 152–160. [Google Scholar] [CrossRef]
- Vitali, L.A.; Dall’Acqua, S.; Maggi, F.; Mártonfi, P.; Papa, F.; Petrelli, D.; Sut, S.; Lupidi, G. Antimicrobial and antioxidant activity of the essential oil from the Carpathian Thymus alternans Klokov. Nat. Prod. Res. 2016, 31, 1–10. [Google Scholar] [CrossRef]
- Pons, M.; Foradada, M.; Estelrich, J. Liposomes obtained by the etanol injection method. Int. J. Pharm. 1993, 95, 51–56. [Google Scholar] [CrossRef]
- Sebaaly, C.; Greige-Gerges, H.; Agusti, G.; Fessi, H.; Charcosset, C. Large-scale preparation of clove essential oil and eugenol-loaded liposomes using a membrane contactor and a pilot plant. J. Liposome Res. 2015, 26, 1–13. [Google Scholar] [CrossRef]
- Sebaaly, C.; Jraij, A.; Fessi, H.; Charcosset, C.; Greige-Gerges, H. Preparation and characterization of clove essential oil-loaded liposomes. Food Chem. 2015, 178, 52–62. [Google Scholar] [PubMed]
- Lee, S.-J.; Han, J.-I.; Lee, G.-S.; Park, M.-J.; Choi, I.-G.; Na, K.-J.; Jeung, E.-B. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol. Pharm. Bull. 2007, 30, 184–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial Activity of Essential Oil of Baccharis dracunculifolia DC (Asteraceae) Aerial Parts at Flowering Period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [PubMed] [Green Version]
- Ryabchenko, B.; Tulupova, E.; Schmidt, E.; Wlcek, K.; Buchbauer, G.; Jirovetz, L. Investigation of Anticancer and Antiviral Properties of Selected Aroma Samples. Nat. Prod. Commun. 2008, 3, 1085–1088. [Google Scholar]
- Ryabchenko, B.; Tulupova, E.; Schmidt, E.; Jäger, W.; Buchbauer, G.; Jirovetz, L. Cytotoxic properties of selected sesquiterpene alcohols on human cervix carcinoma cell lines. J. Essent. Oil Bear. Plants 2011, 5, 316–319. [Google Scholar]





| Substance | CA INCQS 40006 IC50 (µg/mL) | CT INCQS 40042 IC50 (µg/mL) | CK INCQS 40095 IC50 (µg/mL) |
|---|---|---|---|
| Nerolidol | 1000.23 ± 1000.0 | 25,000.29 ± 5000.53 | 15,400.1 ± 1051.39 |
| Nerolidol (L) | 16,000.41 ± 2000.8 | 12,800.27 ± 1112.32 | 47,000.67 ± 12,000.52 |
| Fluconazole | 55.98 ± 12.11 | 1000.99 ± 118.25 | 35.68 ± 1.74 |
| Liposome | 13,000.00 ± 1000.41 | 23,491.13 ± 823.49 | 40,000.75 ± 7000.1 |
| Treatment | CA INCQS 40006 IC50 (µg/mL) | CT INCQS 40042 IC50 (µg/mL) | CK INCQS 40095 IC50 (µg/mL) |
|---|---|---|---|
| Nerolidol + FCZ | 800.86 ± 83.64 | 1000.72 ± 11,303 | 41.93 ± 4.85 |
| Nerolidol (L) + FCZ | 2.56 ± 0.03 | 2.70 ± 0.06 | 72.69 ± 5.62 |
| Fluconazole | 55.98 ± 12.11 | 1000.99 ± 118.25 | 35.68 ± 1.74 |
| Lipossome + FCZ | 788.10 ± 142.15 | 1800.11 ± 164.51 | 61.33 ± 6.75 |
| Formulation | Size (nm) | PI | ZP (mV) |
|---|---|---|---|
| Control liposome | 185.46 ± 3.76 | 0.48 ± 0.01 | −40.9 ± 0.96 |
| Liposomal nerolidol | 132.3 ± 108.3 | 0.42 ±0.02 | −42.6 ± 0.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca Bezerra, C.; de Alencar Júnior, J.G.; de Lima Honorato, R.; dos Santos, A.T.L.; Pereira da Silva, J.C.; Silva, T.G.d.; Leal, A.L.A.B.; de Freitas, T.S.; Vieira, T.A.T.; Esmeraldo Rocha, J.; et al. Antifungal Properties of Nerolidol-Containing Liposomes in Association with Fluconazole. Membranes 2020, 10, 194. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10090194
Fonseca Bezerra C, de Alencar Júnior JG, de Lima Honorato R, dos Santos ATL, Pereira da Silva JC, Silva TGd, Leal ALAB, de Freitas TS, Vieira TAT, Esmeraldo Rocha J, et al. Antifungal Properties of Nerolidol-Containing Liposomes in Association with Fluconazole. Membranes. 2020; 10(9):194. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10090194
Chicago/Turabian StyleFonseca Bezerra, Camila, José Geraldo de Alencar Júnior, Rosilaine de Lima Honorato, Antonia Thassya Lucas dos Santos, Josefa Carolaine Pereira da Silva, Taís Gusmão da Silva, Antonio Linkoln Alves Borges Leal, Thiago Sampaio de Freitas, Thiago Adler Tavares Vieira, Janaína Esmeraldo Rocha, and et al. 2020. "Antifungal Properties of Nerolidol-Containing Liposomes in Association with Fluconazole" Membranes 10, no. 9: 194. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10090194