Beverage–Drug Interaction: Effects of Green Tea Beverage Consumption on Atorvastatin Metabolism and Membrane Transporters in the Small Intestine and Liver of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Green Tea Beverage
2.3. Animals and Treatment
2.4. Determination of ATV and ATV Metabolites in Plasma and Liver
2.5. HPLC/MS Analysis
2.6. CYP Enzyme Activity Assays
2.7. Immunoblotting Analysis
2.8. Determination of Caffeine Levels in Plasma and Liver
2.9. Determination of Fat Contents in the Liver
2.10. Statistical Analysis
3. Results and Discussion
3.1. Constituents of the GT Beverage
3.2. Body Weight, Tissue Weight, and Volume of Drinking Fluid
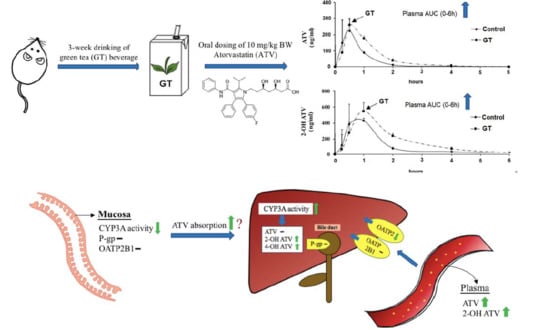
3.3. Pharmacokinetics of ATV after 3 Weeks of GT Consumption in Rats
3.4. Effect of GT Consumption on Hepatic Contents of ATV, 2-OH ATV, and 4-OH ATV
3.5. Effect of GT Comsumption on the Activities of CYP3A and UGT and Protein Expression of Drug Transporters in the Small Intestine
3.6. Effect of GT Comsumption on the Activities of CYP3A and UGT and Protein Expression of Drug Transporters in the Liver
3.7. Effect of GT on Hepatic Contents of Triglycerides and Cholesterol
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, S.; Zhou, H.; Hanson, T.; Yang, L.; Chen, Z.; Zhou, M. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a chinese Cohort of 165,000 adult men. Eur. J. Epidemiol. 2016, 31, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.C.; Chenge, J.; Chen, T. Structural Perspectives of the CYP3A Family and Their Small Molecule Modulators in drug metabolism. Liver Res. 2019, 3, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Ding, X.; Zhang, Q.Y. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm. Sin. B 2016, 6, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Tanigawara, Y. Role of P-glycoprotein in drug disposition. Ther. Drug Monit. 2000, 22, 137–140. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, Z.; Tay-Sontheimer, J.; Levy, R.H.; Ragueneau-Majlessi, I. Intestinal drug interactions mediated by OATPs: A systematic review of preclinical and clinical findings. J. Pharm. Sci USA 2017, 106, 2312–2325. [Google Scholar] [CrossRef] [Green Version]
- Hsiang, B.; Zhu, Y.; Wang, Z.; Wu, Y.; Sasseville, V.; Yang, W.P.; Kirchgessner, T.G. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J. Biol. Chem. 1999, 274, 37161–37168. [Google Scholar] [CrossRef] [Green Version]
- An, G.; Mukker, J.K.; Derendorf, H.; Frye, R.F. Enzyme—And transporter—Mediated beverage-drug interactions: An update on fruit juices and green tea. J. Clin. Pharmacol. 2015, 55, 1313–1331. [Google Scholar] [CrossRef]
- Kimoto, E.; Vourvahis, M.; Scialis, R.J.; Eng, H.; Rodrigues, A.D.; Varma, M.V.S. Mechanistic evaluation of the complex drug-drug interactions of maraviroc: Contribution of Cytochrome P450 3A, P-glycoprotein and organic anion transporting polypeptide 1B1. Drug Metab. Dispos. 2019, 47, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Mallhi, T.H.; Azmi, S.; Azreen, S.A.; Yusra, H.K.; Muhammad, I.Q.; Hamzah, A.A.; Amer, H.K. Effect of fruit/vegetable-drug interactions on CYP450, OATP and p-glycoprotein: A systematic review. Trop. J. Pharm. Res. 2015, 14, 1927–1935. [Google Scholar] [CrossRef]
- Dahan, A.; Altman, H. Food-drug interaction: Grapefruit juice augments drug bioavailability—mechanism, extent and relevance. Eur. J. Clin. Nutr. 2004, 58, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenblatt, D.J. Analysis of drug interactions involving fruit beverages and organic anion-transporting polypeptides. J. Clin. Pharmacol. 2009, 49, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Sohn, O.S.; Surace, A.; Fiala, E.S.; Richie, J.P., Jr.; Colosimo, S.; Zang, E.; Weisburger, J.H. Effects of green and black tea on hepatic xenobiotic metabolizing systems in the male F344 rat. Xenobiotica 1994, 24, 119–127. [Google Scholar] [CrossRef]
- Yao, H.T.; Hsu, Y.R.; Lii, C.K.; Lin, A.H.; Chang, K.H.; Yang, H.T. Effect of commercially available green and black tea beverages on drug-metabolizing enzymes and oxidative stress in Wistar rats. Food Chem. Toxicol. 2014, 70, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Ogawa, S.; Hirobe, R.; Kusunoki, Y.; Kon, R.; Ochiai, W.; Sugiyama, K. High-dose green tea polyphenol intake decreases CYP3A expression in a liver-specific manner with increases in blood substrate drug concentrations. Eur. J. Pharm. Sci. 2016, 89, 137–145. [Google Scholar] [CrossRef]
- Yang, C.S.; Pan, E. The effects of green tea polyphenols on drug metabolism. Expert Opin. Drug Metab. Toxicol. 2012, 8, 677–689. [Google Scholar] [CrossRef]
- Niwattisaiwong, N.; Luo, X.X.; Coville, P.F.; Wanwimolruk, S. Effects of Chinese, Japanese and Western tea on hepatic P450 enzyme activities in rats. Drug Metab. Drug Interact. 2004, 20, 43–56. [Google Scholar] [CrossRef]
- Mirkov, S.; Komoroski, B.J.; Ramírez, J.; Graber, A.Y.; Ratain, M.J.; Strom, S.C.; Innocenti, F. Effects of green tea compounds on irinotecan metabolism. Drug. Metab. Dispos. 2007, 35, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, M.; Ariyoshi, N.; Kotani, A.; Ishii, I.; Nakamura, H.; Nakasa, H.; Ida, M.; Nakamura, H.; Kimura, N.; Kimura, M.; et al. Effects of continuous ingestion of green tea or grape seed extracts on the pharmacokinetics of midazolam. Drug Metab. Pharmacokinet. 2004, 19, 280–289. [Google Scholar] [CrossRef]
- Shin, S.C.; Choi, J.S. Effects of epigallocatechin gallate on the oral bioavailability and pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats. Anticancer Drugs 2009, 20, 584–588. [Google Scholar] [CrossRef]
- Kondo, A.; Narumi, K.; Okuhara, K.; Takahashi, Y.; Furugen, A.; Kobayashi, M.; Iseki, K. Black tea extract and theaflavin derivatives affect the pharmacokinetics of rosuvastatin by modulating organic anion transporting polypeptide (OATP) 2B1 activity. Biopharm. Drug Dispos. 2019, 40, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Albassam, A.A.; Markowitz, J.S. An appraisal of drug-drug interactions with green tea (Camellia sinensis). Planta Med. 2017, 83, 496–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marais, A.D.; Naoumova, R.P.; Firth, J.C.; Penny, C.; Neuwirth, C.K.; Thompson, G.R. Decreased production of low density lipoprotein by atorvastatin after apheresis in homozygous familial hypercholesterolemia. J. Lipid Res. 1997, 38, 2071–2078. [Google Scholar] [PubMed]
- Gibson, D.M.; Stern, R.H.; Abel, R.B.; Whitfield, L.R. Absolute bioavailability of atorvastatin im man. Pharm. Res. (N. Y.) 1997, 14, S253. [Google Scholar]
- Lau, Y.Y.; Okochi, H.; Huang, Y.; Benet, L.Z. Multiple transporters affect the disposition of atorvastatin and its two active hydroxy metabolites: Application of in vitro and ex situ systems. J. Pharmacol. Exp. Ther. 2006, 316, 762–771. [Google Scholar] [CrossRef]
- Grube, M.; Köck, K.; Oswald, S.; Draber, K.; Meissner, K.; Eckel, L.; Böhm, M.; Felix, S.B.; Vogelgesang, S.; Jedlitschky, G.; et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin. Pharmacol. Ther. 2006, 80, 607–620. [Google Scholar] [CrossRef]
- Shitara, Y.; Sugiyama, Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 2006, 112, 71–105. [Google Scholar] [CrossRef]
- Shu, N.; Hu, M.; Liu, C.; Zhang, M.; Ling, Z.; Zhang, J.; Xu, P.; Zhong, Z.; Chen, Y.; Liu, L.; et al. Decreased exposure of atorvastatin in diabetic rats partly due to induction of hepatic Cyp3a and Oatp2. Xenobiotica 2016, 46, 875–881. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Xu, J.; Zhao, K.; Chen, Y.; Liang, L.; Li, P.; Chen, N.; Geng, D.; Zhang, X.; et al. Prediction of atorvastatin pharmacokinetics in high-fat diet and low-dose streptozotocin-induced diabetic rats using a semiphysiologically based pharmacokinetic model involving both enzymes and transporters. Drug Metab. Dispos. 2019, 47, 1066–1079. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Wu, J.J.; Chiang, M.T.; Chang, Y.W.; Chen, J.Y.; Yang, H.T.; Lii, C.K.; Lin, J.H.; Yao, H.T. Determination of the active components and antioxidant activities of commercial tea drinks in Taiwan. J. Food Drug Anal. 2011, 19, 289–308. [Google Scholar]
- Yao, H.T.; Lin, J.H.; Liu, Y.T.; Li, M.L.; Chiang, W. Food-drug interaction between the adlay bran oil and drugs in rats. Nutrients 2019, 11, 2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.T.; Chang, Y.W.; Uramaru, N.; Watanabe, Y.; Kitamura, S.; Kuo, Y.H.; Lii, C.K.; Yeh, T.K. Effects of Bu-Zhong-Yi-Qi-Tang on hepatic drug-metabolizing enzymes and plasma tolbutamide concentration in rats. J. Ethnopharmacol. 2012, 142, 121–128. [Google Scholar] [CrossRef]
- Lad, R. Validation of individual quantitative methods for determination of cytochrome P450 probe substrates in human dried blood spots with HPLC-MS/MS. Bioanalysis 2010, 2, 1849–1861. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Carlson, S.E.; Goldfarb, S. A sensitive enzymatic method for determination of free and esterified tissue cholesterol. Clin. Chim. Acta 1977, 79, 575–582. [Google Scholar] [CrossRef]
- Grabowski, T.; Jaroszewski, J.J.; Borucka, B.; Ziółkowski, H. C (max) and t (max) verification using Fibonacci sequence and absorption rate. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 131–138. [Google Scholar] [CrossRef]
- McFeely, S.J.; Wu, L.; Ritchie, T.K.; Unadkat, J. Organic anion transporting polypeptide 2B1-More than a glass-full of drug interactions. Pharmacol. Ther. 2019, 196, 204–215. [Google Scholar] [CrossRef]
- Roth, M.; Timmermann, B.N.; Hagenbuch, B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab. Dispos. 2011, 39, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Roncal-Jimenez, C.A.; Lanaspa, M.A.; Rivard, C.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Jalal, D.; Andres-Hernando, A.; Tanabe, K.; Madero, M.; Li, N.; et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism 2011, 60, 1259–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, L.P.; Teel, R.W. Effects of high sucrose diet on body and liver weight and hepatic enzyme content and activity in the rat. In Vivo 2003, 17, 61–65. [Google Scholar] [PubMed]





| Gallic Acid (GA) | Epigallocatechin (EGC) | Epigallocatechin Gallate (EGCG) | Epicatechin (EC) | Gallocatechin Gallate (GCG) | Epicatechin Gallate (ECG) | Total Catechins 2 | |
|---|---|---|---|---|---|---|---|
| Polyphenols (μg/mL) | 3.8 | 282.6 | 208.8 | 62.9 | 45.6 | 67.0 | 670.7 |
| Sugars (g/L) | Glucose 7.1 | Sucrose 38.9 | Fructose 9.6 | Total sugars 3 55.6 | |||
| AUC (0-t) (ng/mLxh) | Cmax (ng/mL) | Tmax (min) | |
|---|---|---|---|
| ATV | |||
| Control group | 176.5 ± 101.1 | 235.5 ± 136.1 | 27.0 ± 6.7 |
| GT group | 326.8 ± 88.7 * | 307.4 ± 157.7 | 33.0 ± 16.4 |
| 2-OH ATV | |||
| Control group | 548.3 ± 134.2 | 466.2 ± 225.7 | 48.0 ± 16.4 |
| GT group | 1059.8 ± 185.0 * | 557.5 ± 125.7 | 72.0 ± 26.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.-T.; Hsu, Y.-R.; Li, M.-L. Beverage–Drug Interaction: Effects of Green Tea Beverage Consumption on Atorvastatin Metabolism and Membrane Transporters in the Small Intestine and Liver of Rats. Membranes 2020, 10, 233. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10090233
Yao H-T, Hsu Y-R, Li M-L. Beverage–Drug Interaction: Effects of Green Tea Beverage Consumption on Atorvastatin Metabolism and Membrane Transporters in the Small Intestine and Liver of Rats. Membranes. 2020; 10(9):233. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10090233
Chicago/Turabian StyleYao, Hsien-Tsung, Ya-Ru Hsu, and Mei-Ling Li. 2020. "Beverage–Drug Interaction: Effects of Green Tea Beverage Consumption on Atorvastatin Metabolism and Membrane Transporters in the Small Intestine and Liver of Rats" Membranes 10, no. 9: 233. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10090233