Bone Regeneration Assessment of Polycaprolactone Membrane on Critical-Size Defects in Rat Calvaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the Polycaprolactone Membrane
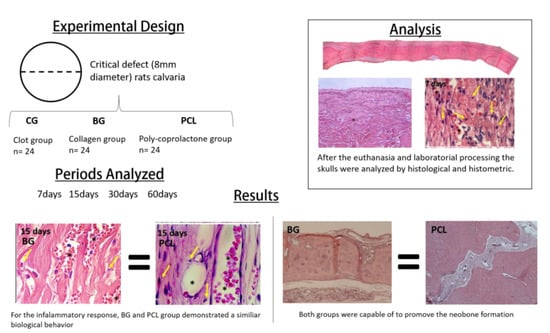
2.2. Samples
- Clot group (CG) (negative control): The critical bone defect was filled with blood clot.
- Porcine collagen group (Bio-Guide®) (BG) (positive control): The critical bone defect was filled with blood clot and a porcine collagen membrane was placed on the defect.
- PCL group with 5% HA (PCL) (experimental group): The critical bone defect was filled with blood clot and a PCL membrane with 5% HA was placed on the defect.
2.3. Experimental Surgical Procedure
2.4. Histological and Histometric Analysis
2.5. Statistical Analysis
3. Results
3.1. Membranes’ Osteopromotive Histological Behavior
3.2. Inflammatory Response: Lymphocyte and Vessel Counting
3.3. Histometry: Newly Formed Bone Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buser, D. 20 Years of Guided Bone Regenration in Implant Dentistry; Quintessence: Chicago, IL, USA, 2010; pp. 25–41. [Google Scholar]
- Noelken, R.; Pausch, T.; Wagner, W.; Al-Nawas, B. Peri-implant defect graftinh with autogenous bone or bone graft material in immediate implant placemente in molar extraction sites-1- to 3-year results of a prospective randomized study. Clin. Oral Implant. Res. 2020, 31, 1138–1148. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hwang, K.G.; Jun, S.H.; Tallarico, M.; Kwon, A.M.; Park, C.J. Radiologic compartive analysis between saline and platlet-rich fibrin filling after hydraulic transcrestal sinus lifting without adjunctive bone graft: A randomized controlled trial. Clin. Oral Implant. Res. 2020, 31, 1087–1093. [Google Scholar] [CrossRef]
- De Bruyckere, T.; Cabeza, R.G.; Eghbali, A.; Younes, F.; Cleymaet, R.; Cosyn, J. A randomized controlled study comparing guided bone regeneration with conncective tissue graft to reestablish buccal convexity at implant sites: A 1-year volumetric alanysis. Clin. Implant. Dent. Res. 2020, 22, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A., Jr.; Savadori, P.; Barlattani, A.; Franco, R.; Michele, M.; Gianfreda, F.; Bollero, P. Autologous tooth graft: A histological comparison between dentin mixed with xenograft and dentin alone grafts in socket preservation. J. Biol. Regul. Homeost. Agents 2020, 33, 189–197. [Google Scholar]
- Zhao, X.; Zou, L.; Chen, Y.; Tang, Z. Staged horizontal bone augmentation for dental implants in aesthetic zones: A prospective randomized controlled clinical trial comparing a half-columnar bone block harvested from the ramus versus a rectangular bone block from the symphysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 1326–1334. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- AlKanan, A.; Greenwell, H.; Patel, A.; Hill, M.; Shumway, B.; Lowy, J. Ridge Preservation Comparing the Clinical and Histologic Healing of Membrane vs No-Membrane Approach to Buccal Overlay Grafting. Int. J. Periodontics Restor. Dent. 2019, 39, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.I.; Eisner, B.M.; Jung, R.E.; Basler, T.; Schneider, D.; Hammerle, C.H.F. Hard tissue changes afeter guided bone regeneration of peri-implant defects comparing blocks versus particulate bone substitutes: 6-months results of a randomized controlled clinical trial. Clin. Oral Implant. Res. 2019, 30, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Petsos, H.; Ratka-Krüger, P.; Neukranz, E.; Raetzke, P.; Eickholz, P.; Nickles, K. Infrabony defects 20 years after open flap debridement and guided tissue regeneration. J. Clin. Periodontol. 2019, 46, 552–563. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; Novaes, A.; Bustamanti, A.; Villavicencio, J.; Muller, E.; Pulido, J. Supportive periodontal therapy in South America. A retrospective multi-practice study on compliance. J. Periodontol. 1999, 73, 301–306. [Google Scholar] [CrossRef]
- Bostman, O. Osteolytic changes accompanying degradation of absorbable fracture fixation implants. J. Bone Jt. Surg. Br. Vol. 1991, 73, 679–682. [Google Scholar] [CrossRef]
- Atef, M.; Tarek, A.; Shaheen, M.; AlArawi, R.M.; Askar, N. Horizontal ridge augmentation using native collagen membrane vs titanium mesh in atrophic maxillary ridges: Randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2020, 22, 156–166. [Google Scholar] [CrossRef]
- Koo, T.H.; Song, Y.W.; Cha, J.K.; Jung, U.W.; Kim, C.S.; Lee, J.S. Histologic analysis following grafting of damaged extraction sockets using deproteinized bovine or procine bone mineral: A randomized clinical trial. Clin. Oral Implant. Res. 2020, 31, 93–102. [Google Scholar] [CrossRef]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and histomorphometric analysis of bone tissue after guided bone regeneration with non-resorbable membranes vs resorbable membranes and titanium mesh. Clin. Implant. Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, D.; Luz, D.; Moraschini, V.; Rodrigues, D.M.; Barboza, E.S.P. Alveolar rigde preservation using a non-resorbable membrane: Randomized clinical trial with biomolecular analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 1465–1473. [Google Scholar] [CrossRef]
- Neto, W.A.R.; Pereira, I.H.L.; Ayres, E.; De Paula, A.C.C.; Averous, L.; Góes, A.M.; Oréfice, R.L.; Bretas, R.E.S. Influence of the microstructure and mechanical strength of nanofibers of biodegradable polymers with hydroxyapatite in stem cells growth. Electrospinning, characterization and cell viability. Polym. Degrad. Stab. 2012, 97, 2037–2051. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer Scaffolds for Biomaterials Applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- De Oliveria Puttini, I.; Poli, P.P.; Maiorana, C.; de Vasconcelos, I.R.; Schmidt, L.E.; Colombo, L.T.; Hadad, H.; Mulinaridos Santos, G.; de Carvalho, P.S.P.; Souza, F. Ávila Evaluation of Osteoconduction of Biphasic Calcium Phosphate Ceramic in the Calvaria of Rats: Microscopic and Histometric Analysis. J. Funct. Biomater. 2019, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, L.E.; Hadad, H.; De Vasconcelos, I.R.; Colombo, L.T.; Da Silva, R.C.; Santos, A.F.P.; Cervantes, L.C.C.; Poli, P.P.; Signorino, F.; Maiorana, C.; et al. Critical Defect Healing Assessment in Rat Calvaria Filled with Injectable Calcium Phosphate Cement. J. Funct. Biomater. 2019, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, L.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Belgacem, M.; Gandini, A. Monomers, Polymers and Composites from Renewable Resources; Elsever: Amsterdan, The Netherlands, 2008; pp. 94–112. [Google Scholar]
- Lee, P.; Tran, K.; Chang, W.; Shelke, N.B.; Kumbar, S.G.; Yu, X. Influence of chondroitin sulfate and hyaluronic acid presence in nanofibers and its alignment on the bone marrow stromal cells: Cartilage regeneration. J. Biomed. Nanotechnol. 2014, 10, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar] [CrossRef] [Green Version]
- Gishto, A.; Farrell, K.; Kothapalli, C.R. Tuning composition and architecture of biomimetic scaffolds for enhanced matrix synthesis by murine cardiomyocytes. J. Biomed. Mater. Res. Part A 2014, 103, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Leung, M.; Wang, X.; Chang, J.Y.F.; Tsao, C.T.; Sham, J.G.C.; Edmondson, D.; Zhang, M. Bi-layer scaffold of chitosan/PCL-nanofibrous mat and PLLA-microporous disc for skin tissue engineering. J. Biomed. Nanotechnol. 2014, 10, 1105–1113. [Google Scholar] [CrossRef]
- Auras, R.; Lim, L.; Selke, S.; Tsuji, H. Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; Wiley and sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Kim, J.H.; Choung, P.-H.; Kim, I.Y.; Lim, K.T.; Son, H.M.; Choung, Y.-H.; Cho, C.-S.; Chung, J.H. Electrospun nanofibers composed of poly(ε-caprolactone) and polyethylenimine for tissue engineering applications. Mater. Sci. Eng. C 2009, 29, 1725–1731. [Google Scholar] [CrossRef]
- Bae, G.Y.; Jang, J.; Jeong, Y.G.; Lyoo, W.S.; Gil Min, B. Superhydrophobic PLA fabrics prepared by UV photo-grafting of hydrophobic silica particles possessing vinyl groups. J. Colloid Interface Sci. 2010, 344, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Frey, M.W.; Baeumner, A.J. Electrospun polylactic acid nanofiber membranes as substrates for biosensor assemblies. J. Membr. Sci. 2006, 279, 354–363. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kamada, F.; Kawazoe, N.; Tateishi, T.; Chen, G. Structural changes and biodegradation of PLLA, PCL, and PLGA sponges during in vitro incubation. Polym. Eng. Sci. 2010, 50, 1895–1903. [Google Scholar] [CrossRef]
- Cho, S.J.; Jung, S.M.; Kang, M.; Shin, H.S.; Youk, J.H. Preparation of hydrophilic PCL nanofiber scaffolds via electrospinning of PCL/PVP-b-PCL block copolymers for enhanced cell biocompatibility. Polymer 2015, 69, 95–102. [Google Scholar] [CrossRef]
- Dong, C.; Guo, Y.; Qiu, K.; Gu, Z.; Feng, X. In vitro degradation and controlled release behavior of D,L-PLGA50 and PCL-b-D,L-PLGA50 copolymer microspheres. J. Control Release 2005, 107, 53–64. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Xie, C.; Zhuang, H.; Zhou, S.; Weng, J. Hydroxyapatite nucleation and growth mechanism on electrospun fibers functionalized with different chemical groups and their combinations. Biomaterials 2010, 31, 4620–4629. [Google Scholar] [CrossRef]
- Nirmala, R.; Nam, K.T.; Park, D.K.; Woo-Il, B.; Navamathavan, R.; Kim, H.Y. Structural, thermal, mechanical and bioactivity evaluation of silver-loaded bovine bone hydroxyapatite grafted poly(ε-caprolactone) nanofibers via electrospinning. Surf. Coat. Technol. 2010, 205, 174–181. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Wutticharoenmongkol, P.; Pavasant, P.; Supaphol, P. Osteoblastic Phenotype Expression of MC3T3-E1 Cultured on Electrospun Polycaprolactone Fiber Mats Filled with Hydroxyapatite Nanoparticles. Biomacromolecules 2007, 8, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.W.; Lee, H.-H.; Knowles, J.C. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly(lactic acid) for bone regeneration. J. Biomed. Mater. Res. Part A 2006, 79, 643–649. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Low, S.; Choon, A.T.; Kumar, A.B.; Ramakrishna, S. Electrospun-modified nanofibrous scaffolds for the mineralization of osteoblast cells. J. Biomed. Mater. Res. Part A 2008, 85, 408–417. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric Nanofibers in Tissue Engineering. Tissue Eng. Part B Rev. 2011, 17, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Wooley, P.H.; Yang, S.Y. Biocompatibility of Poly-epsilon-caprolactone-hydroxyapatite composite on mouse bone marrow-derived osteoblasts and endothelial cells. J. Orthop. Surg. Res. 2009, 26, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, W.A.R.; De Paula, A.C.C.; Martins, T.M.M.; Goes, A.M.; Averous, L.; Schlatter, G.; Bretas, R.E.S. Poly (butylene adipate-co-terephthalate)/hydroxyapatite composite structures for bone tissue recovery. Polym. Degrad. Stab. 2015, 120, 61–69. [Google Scholar] [CrossRef]
- Hidalgo Pitaluga, L.; Trevelin Souza, M.; Dutra Zanotto, E.; Santocildes Romero, M.E.; Hatton, P.V. Electrospun F18 Bioactive Glass/PCL-Poly (ε-caprolactone)-Membrane for Guided Tissue Regeneration. Materials 2018, 11, 400. [Google Scholar] [CrossRef] [Green Version]
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Bizenjima, T.; Takeuchi, T.; Seshima, F.; Saito, A. Effect of poly (lactide-co-glycolide) (PLGA)-coated beta-tricalcium phosphate on the healing of rat calvarial bone defects: A comparative study with pure-phase beta-tricalcium phosphate. Clin. Oral Implant. Res. 2016, 27, 1360–1367. [Google Scholar] [CrossRef]
- de Freitas Silva, L.; de Carvalho Reis, E.N.R.; Barbara, T.A.; Bonardi, J.P.; Garcia, I.R.; De Carvalho, P.S.P.; Ponzoni, D. Assessment of bone repair in critical-size defect in the calvarium of rats after the implantation of tricalcium phosphate beta (β-TCP). Acta Histochem. 2017, 119, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassi, A.P.F.; Bizelli, V.F.; Brasil, L.F.D.M.; Pereira, J.C.; Al-Sharani, H.M.; Momesso, G.A.C.; Faverani, L.P.; Lucas, F.D.A. Is the Bacterial Cellulose Membrane Feasible for Osteopromotive Property? Membranes 2020, 10, 230. [Google Scholar] [CrossRef]
- Danieletto-Zanna, C.F.; Bizelli, V.F.; Ramires, G.A.D.A.; Francatti, T.M.; De Carvalho, P.S.P.; Bassi, A.P.F. Osteopromotion Capacity of Bovine Cortical Membranes in Critical Defects of Rat Calvaria: Histological and Immunohistochemical Analysis. Int. J. Biomater. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hassumi, J.S.; Mulinari-Santos, G.; Fabris, A.L.D.S.; Jacob, R.G.M.; Gonçalves, A.; Rossi, A.C.; Freire, A.R.; Faverani, L.P.; Okamoto, R. Alveolar bone healing in rats: Micro-CT, immunohistochemical and molecular analysis. J. Appl. Oral Sci. 2018, 26, e20170326. [Google Scholar] [CrossRef]
- Ramalho-Ferreira, G.; Faverani, L.P.; Prado, F.B.; Garcia, I.R., Jr.; Okamoto, R. Raloxifene enhances peri-implant bone healing in osteoporotic rats. Int. J. Oral Maxillofac. Surg. 2015, 44, 798–805. [Google Scholar] [CrossRef]
- Faverani, L.P.; Polo, T.O.B.; Ramalho-Ferreira, G.; Momesso, G.A.C.; Hassumi, J.S.; Rossi, A.C.; Freire, A.R.; Prado, F.B.; Luvizuto, E.R.; Gruber, R.; et al. Raloxifene but not alendronate can compensate the impaired osseointegration in osteoporotic rats. Clin. Oral Investig. 2017, 22, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Vajgel, A.; Mardas, N.; Farias, B.C.; Petrie, A.; Cimões, R.; Dono, N. A systematic review on the critical size defecet model. Clin. Oral Implant. Res. 2014, 25, 879–893. [Google Scholar] [CrossRef]
- Yogui, F.C.; Momesso, G.A.C.; Faverani, L.P.; Polo, T.O.B.; Ramalho-Ferreira, G.; Hassumi, J.S.; Rossi, A.C.; Freire, A.R.; Prado, F.B.; Okamoto, R. A SERM increasing the expression of the osteoblastogenesis and mineralization-related proteins and improving quality of bone tissue in an experimental model of osteoporosis. J. Appl. Oral Sci. 2018, 26, e20170329. [Google Scholar] [CrossRef]
- De Oliveira, D.; Hassumi, J.S.; Gomes-Ferreira, P.H.D.S.; Polo, T.O.B.; Ferreira, G.R.; Faverani, L.P.; Okamoto, R. Short term sodium alendronate administration improves the peri-implant bone quality in osteoporotic animals. J. Appl. Oral Sci. 2017, 25, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalho-Ferreira, G.; Faverani, L.P.; Momesso, G.A.C.; Luvizuto, E.R.; de Oliveira Puttini, I.; Okamoto, R. Effect of antiresorptive drugs in the alveolar bone healing. A histometric and immunohistochemical study in ovariectomized rats. Clin. Oral Investig. 2017, 21, 1485–1494. [Google Scholar] [CrossRef]
- Ramalho-Ferreira, G.; Faverani, L.P.; Grossi-Oliveira, G.A.; Okamoto, T.; Okamoto, R. Alveolar bone dynamics in osteoporotic rats treated with raloxifene or alendronate: Confocal microscopy analysis. J. Biomed. Opt. 2015, 20, 38003. [Google Scholar] [CrossRef] [PubMed]
- Messora, M.R.; Nagata, M.J.H.; Dornelles, R.C.M.; Bomfim, S.R.M.; Furlaneto, F.A.C.; De Melo, L.G.N.; Deliberador, T.M.; Bosco, A.F.; Garcia, V.G.; Fucini, S.E. Bone healing in critical-size defects treated with platelet-rich plasma activated by two different methods. A histologic and histometric study in rat calvaria. J. Periodontal Res. 2008, 43, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Messora, M.R.; Nagata, M.J.H.; Dornelles, R.C.M.; Bomfim, S.R.M.; Fucini, S.E.; Garcia, V.G.; Bosco, A.F. Bone healing in critical-size defects treated with platelet-rich palsma: A histologic and histometric study in rat calvaria. J. Periodontal Res. 2008, 43, 217–223. [Google Scholar] [CrossRef]
- Célio-Mariano, R.; Messora, M.R.; De Morais, A.; Nagata, M.; Furlaneto, F.A.C.; Avelino, C.; Paula, F.; Ferreira, S.; Pinheiro, M.; De Sene, J.P. Bone healing in critical-size defects treated with platelet-rich plasma: A histologic and histometric study in the calvaria of diabetic rat. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 72–78. [Google Scholar] [CrossRef]
- Araújo, C.R.G.; Astarita, C.; D’Aquino, R.; Pelegrine, A.A. Evaluation of Bone Regeneration in Rat Calvaria Using Bone Autologous Micrografts and Xenografts: Histological and Histomorphometric Analysis. Materials 2020, 13, 4284. [Google Scholar] [CrossRef]
- Ruckh, T.T.; Kumar, K.; Kipper, M.J.; Popat, K.C. Osteogenic differentiation of bone marrow stromal cells on poly(epsilon-caprolactone) nanofiber scaffolds. Acta Biomater. 2010, 6, 2946–2959. [Google Scholar] [CrossRef]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J. Biomed. Mater. Res. Part A 2010, 94, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Kulanthaivel, S.; Mishra, U.; Agarwal, T.; Giri, S.; Pal, K.; Pramanik, K.; Banerjee, I. Improving the osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceram. Int. 2015, 41, 11323–11333. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chang, Y.-S. Preparation and characterization of composite nanofibers of polycaprolactone and nanohydroxyapatite for osteogenic differentiation of mesenchymal stem cells. Colloids Surf. B Biointerfac. 2011, 86, 169–175. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Barradas, A.M.C.; van Blitterswijk, C.A.; de Boer, J.; Feijen, J.; Grijpma, D.W. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010, 6, 4207–4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Wan, Y.; Dalai, S.; Zhang, R. Response of rat osteoblasts to polycaprolactone/chitosan blend porous scaffolds. J. Biomed. Mater. Res. Part. A 2010, 92, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Hutamacher, D.; Kirsch, A.; Ackermann, K.; Huerzeter, M. Processing and Fabrication of bioresorbable implants and devices e state of the art and future perspective. In Processing and Fabrication of Advanced Materials; World Scientific: Singapore, 1998. [Google Scholar]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 274, 124–174. [Google Scholar] [CrossRef] [Green Version]
- Dikici, B.A.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A Novel Bilayer Polycaprolactone Membrane for Guided Bone Regeneration: Combining Electrospinning and Emulsion Templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassi, A.P.F.; Bizelli, V.F.; Francatti, T.M.; Rezende de Moares Ferreira, A.C.; Carvalho Pereira, J.; Al-Sharani, H.M.; de Almeida Lucas, F.; Faverani, L.P. Bone Regeneration Assessment of Polycaprolactone Membrane on Critical-Size Defects in Rat Calvaria. Membranes 2021, 11, 124. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11020124
Bassi APF, Bizelli VF, Francatti TM, Rezende de Moares Ferreira AC, Carvalho Pereira J, Al-Sharani HM, de Almeida Lucas F, Faverani LP. Bone Regeneration Assessment of Polycaprolactone Membrane on Critical-Size Defects in Rat Calvaria. Membranes. 2021; 11(2):124. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11020124
Chicago/Turabian StyleBassi, Ana Paula Farnezi, Vinícius Ferreira Bizelli, Tamires Mello Francatti, Ana Carulina Rezende de Moares Ferreira, Járede Carvalho Pereira, Hesham Mohammed Al-Sharani, Flavia de Almeida Lucas, and Leonardo Perez Faverani. 2021. "Bone Regeneration Assessment of Polycaprolactone Membrane on Critical-Size Defects in Rat Calvaria" Membranes 11, no. 2: 124. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11020124