Phenolphthalein Anilide Based Poly(Ether Sulfone) Block Copolymers Containing Quaternary Ammonium and Imidazolium Cations: Anion Exchange Membrane Materials for Microbial Fuel Cell
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of the –OH Terminated Telechelic Block, PES-1
2.3. Synthesis of the F-Terminated Telechelic Block, PES-3
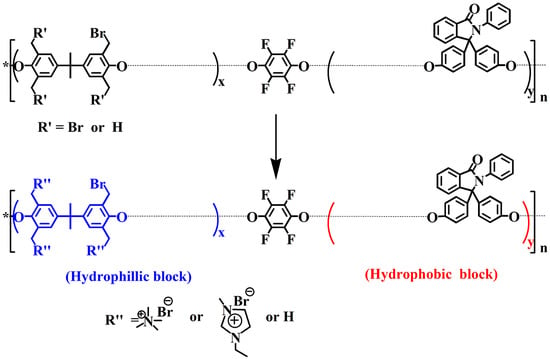
2.4. Synthesis of the Precursor Multiblock Copolymer, Cardo-PES
2.5. Synthesis of the Benzyl Brominated Multiblock Copolymer, PES-Br
2.6. Synthesis of Cardo-PES-QA and Cardo-PES-IM
2.7. Fabrication of Membranes
2.8. Characterization
2.9. Microbial Fuel Cell
3. Results and Discussions
3.1. Synthesis and Characterizations of the Oligomers and the Precursor Multiblock Copolymer
3.2. Synthesis and Characterization of the Brominated and Quaternized Polymers
3.3. Thermal Stability
3.4. Morphology
3.5. Membrane Properties
3.6. Microbial Fuel Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Kundu, P. Biocatalysts in Microbial Fuel Cells. Enzym. Microb. Technol. 2010, 47, 179–188. [Google Scholar] [CrossRef]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q. Recent Advances in the Separators for Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ledin, P.A.; Shevchenko, V.V.; Tsukruk, V.V. Architecture, Assembly, and Emerging Applications of Branched Functional Polyelectrolytes and Poly(Ionic Liquid)s. ACS Appl. Mater. Interfaces 2015, 7, 12570–12596. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Khilari, S.; Bera, K.; Pradhan, D.; Das, D. Application of PVA–PDDA Polymer Electrolyte Composite Anion Exchange Membrane Separator for Improved Bioelectricity Production in a Single Chambered Microbial Fuel Cell. Chem. Eng. J. 2014, 257, 138–147. [Google Scholar] [CrossRef]
- Lin, C.X.; Wu, H.Y.; Li, L.; Wang, X.Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Anion Conductive Triblock Copolymer Membranes with Flexible Multication Side Chain. ACS Appl. Mater. Interfaces 2018, 10, 18327–18337. [Google Scholar] [CrossRef]
- Rao, A.H.N.; Nam, S.Y.; Kim, T.-H. Comb-Shaped Alkyl Imidazolium-Functionalized Poly(Arylene Ether Sulfone)s as High Performance Anion-Exchange Membranes. J. Mater. Chem. A 2015, 3, 8571–8580. [Google Scholar] [CrossRef]
- Gu, S.; Cai, R.; Yan, Y. Self-Crosslinking for Dimensionally Stable and Solvent-Resistant Quaternary Phosphonium Based Hydroxide Exchange Membranes. Chem. Commun. 2011, 47, 2856–2858. [Google Scholar] [CrossRef]
- Zhao, C.H.; Gong, Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M. Self-Crosslinked Anion Exchange Membranes by Bromination of Benzylmethyl-Containing Poly(Sulfone)s for Direct Methanol Fuel Cells. Int. J. Hydrog. Energy 2012, 37, 11383–11393. [Google Scholar] [CrossRef]
- Morandi, C.G.; Peach, R.; Krieg, H.M.; Kerres, J. Novel Morpholinium-Functionalized Anion-Exchange PBI–Polymer Blends. J. Mater. Chem. A 2014, 3, 1110–1120. [Google Scholar] [CrossRef]
- Niu, M.; Zhang, C.; He, G.; Zhang, F.; Wu, X. Pendent Piperidinium-Functionalized Blend Anion Exchange Membrane for Fuel Cell Application. Int. J. Hydrog. Energy 2019, 44, 15482–15493. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Devaraju, S.; Kim, N.; Paik, H.-J. Synthesis and Characterization of Poly(Ether Sulfone) Block Copolymers Containing Pendent Quaternary Ammonium- and Imidazolium Groups as Anion Exchange Membranes. Solid State Ion. 2018, 314, 46–56. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Sen, S.K.; Ghosh, A.; Maji, S.; Banerjee, S. Synthesis, Characterization, and Comparison of Properties of New Fluorinated Poly(Arylene Ether)s Containing Phthalimidine Moiety in the Main Chain. Polym. Adv. Technol. 2009, 21, 767–773. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukasawa, K.; Nishino, E.; Yamaguchi, S.; Yamada, K.; Tanaka, H.; Bae, B.; Miyatake, K.; Watanabe, M. Anion Conductive Block Poly(Arylene Ether)s: Synthesis, Properties, and Application in Alkaline Fuel Cells. J. Am. Chem. Soc. 2011, 133, 10646–10654. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y. Quaternized Polyethersulfone Cardo Anion Exchange Membranes for Direct Methanol Alkaline Fuel Cells. J. Membr. Sci. 2005, 262, 1–4. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zeng, Q.H. Quaternized Cardo Polyetherketone Anion Exchange Membrane for Direct Methanol Alkaline Fuel Cells. J. Power Sources 2009, 193, 541–546. [Google Scholar] [CrossRef]
- Tanaka, M.; Koike, M.; Miyatake, K.; Watanabe, M. Synthesis and Properties of Anion Conductive Ionomers Containing Fluorenyl Groups for Alkaline Fuel Cell Applications. Polym. Chem. 2010, 2, 99–106. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Q.; Wang, J.; Zhang, S.; Li, S. Synthesis and Alkaline Stability of Novel Cardo Poly(Aryl Ether Sulfone)s with Pendent Quaternary Ammonium Aliphatic Side Chains for Anion Exchange Membranes. Polymer 2010, 51, 5407–5416. [Google Scholar] [CrossRef]
- Rao, A.H.; Kim, H.-J.; Nam, S.; Kim, T.-H. Cardo Poly(Arylene Ether Sulfone) Block Copolymers with Pendant Imidazolium Side Chains as Novel Anion Exchange Membranes for Direct Methanol Alkaline Fuel Cell. Polymer 2013, 54, 6918–6928. [Google Scholar] [CrossRef]
- Badami, A.S.; Lane, O.; Lee, H.-S.; Roy, A.; McGrath, J.E. Fundamental Investigations of the Effect of the Linkage Group on the Behavior of Hydrophilic–Hydrophobic Poly(Arylene Ether Sulfone) Multiblock Copolymers for Proton Exchange Membrane Fuel Cells. J. Membr. Sci. 2009, 333, 1–11. [Google Scholar] [CrossRef]
- Myung, B.Y.; Kim, J.S.; Kim, J.-J.; Yoon, T.H. Synthesis and Characterization of Novel Polyimides with 2,2-bis[4(4-Aminophenoxy)Phenyl]Phthalein-3?,5?-Bis(Trifluoromethyl)Anilide. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3361–3374. [Google Scholar] [CrossRef]
- Zhu, L.; Pan, J.; Wang, Y.; Han, J.; Zhuang, L.; Hickner, M.A. Multication Side Chain Anion Exchange Membranes. Macromolecules 2016, 49, 815–824. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A. Degradation of Imidazolium- and Quaternary Ammonium-Functionalized Poly(Fluorenyl Ether Ketone Sulfone) Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2012, 4, 5775–5781. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Rowlett, J.R.; McGrath, J.E.; Mack, N.H.; Kim, Y.S. Controlled Disulfonated Poly(Arylene Ether Sulfone) Multiblock Copolymers for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 5779–5788. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Mistri, E.A.; Banerjee, S.; Komber, H.; Voit, B. Highly Fluorinated Sulfonated Poly(Arylene Ether Sulfone) Copolymers: Synthesis and Evaluation of Proton Exchange Membrane Properties. Ind. Eng. Chem. Res. 2013, 52, 2772–2783. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Qu, C. Imidazolium Functionalized Polysulfone Anion Exchange Membrane for Fuel Cell Application. J. Mater. Chem. 2011, 21, 12744–12752. [Google Scholar] [CrossRef]
- Hibbs, M.R.; Fujimoto, C.H.; Cornelius, C.J. Synthesis and Characterization of Poly(Phenylene)-Based Anion Exchange Membranes for Alkaline Fuel Cells. Macromolecules 2009, 42, 8316–8321. [Google Scholar] [CrossRef]
- Yan, X.; He, G.; Gu, S.; Wu, X.; Du, L.; Wang, Y. Imidazolium-Functionalized Polysulfone Hydroxide Exchange Membranes for Potential Applications in Alkaline Membrane Direct Alcohol Fuel Cells. Int. J. Hydrogen Energy 2012, 37, 5216–5224. [Google Scholar] [CrossRef]
- Hossain, A.; Lim, Y.; Lee, S.; Jang, H.; Choi, S.; Jeon, Y.; Lim, J.; Kim, W.G. Comparison of Alkaline Fuel Cell Membranes of Random & Block Poly(Arylene Ether Sulfone) Copolymers Containing Tetra Quaternary Ammonium Hydroxides. Int. J. Hydrogen Energy 2014, 39, 2731–2739. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Yu, Y.; Meng, Y. Synthesis and Properties of Multiblock Ionomers Containing Densely Functionalized Hydrophilic Blocks for Anion Exchange Membranes. J. Membr. Sci. 2014, 467, 1–12. [Google Scholar] [CrossRef]
- Lai, A.N.; Wang, L.S.; Lin, C.X.; Zhuo, Y.Z.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Phenolphthalein-Based Poly(Arylene Ether Sulfone Nitrile)s Multiblock Copolymers As Anion Exchange Membranes for Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2015, 7, 8284–8292. [Google Scholar] [CrossRef]
- Weiber, E.A.; Meis, D.; Jannasch, P. Anion Conducting Multiblock Poly(Arylene Ether Sulfone)s Containing Hydrophilic Segments Densely Functionalized with Quaternary Ammonium Groups. Polym. Chem. 2015, 6, 1986–1996. [Google Scholar] [CrossRef]
- Kwasny, M.T.; Zhu, L.; Hickner, M.A.; Tew, G.N. Thermodynamics of Counterion Release Is Critical for Anion Exchange Membrane Conductivity. J. Am. Chem. Soc. 2018, 140, 7961–7969. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Qiu, L.; Qiu, B.; Peng, Y.; Yan, F. A Soluble and Conductive Polyfluorene Ionomer with Pendant Imidazolium Groups for Alkaline Fuel Cell Applications. Macromolecules 2011, 44, 9642–9649. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Li, S.; Zhang, S. Synthesis of Multi-Block Poly(Arylene Ether Sulfone) Copolymer Membrane with Pendant Quaternary Ammonium Groups for Alkaline Fuel Cell. J. Power Sources 2011, 196, 4445–4450. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Wang, C.; Lee, Y.M.; Guiver, M.D. Phenyltrimethylammonium Functionalized Polysulfone Anion Exchange Membranes. Macromolecules 2012, 45, 2411–2419. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Liu, Q.; Meng, Y. Synthesis and Properties of Anion Conductive Ionomers Containing Tetraphenyl Methane Moieties. ACS Appl. Mater. Interfaces 2012, 4, 3627–3635. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Yu, Y.; Meng, Y. Quaternized Poly(Arylene Ether) Ionomers Containing Triphenyl Methane Groups for Alkaline Anion Exchange Membranes. J. Mater. Chem. A 2013, 1, 4324–4335. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Liu, Q.; Meng, Y. Synthesis and Properties of Anion Conductive Multiblock Copolymers Containing Tetraphenyl Methane Moieties for Fuel Cell Application. J. Membr. Sci. 2013, 436, 202–212. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Shen, K.; Feng, P.; Zhang, Y.; Xu, X.; Hu, W.; Jiang, Z.; Guiver, M.D. Naphthalene-Based Poly(Arylene Ether Ketone) Anion Exchange Membranes. J. Mater. Chem. A 2013, 1, 6481. [Google Scholar] [CrossRef]
- Park, D.-Y.; Kohl, P.A.; Beckham, H.W. Anion-Conductive Multiblock Aromatic Copolymer Membranes: Structure–Property Relationships. J. Phys. Chem. C 2013, 117, 15468–15477. [Google Scholar] [CrossRef]
- Rao, A.H.; Thankamony, R.L.; Kim, H.-J.; Nam, S.; Kim, T.-H. Imidazolium-Functionalized Poly(Arylene Ether Sulfone) Block Copolymer as an Anion Exchange Membrane for Alkaline Fuel Cell. Polymer 2013, 54, 111–119. [Google Scholar] [CrossRef]
- Jasti, A.; Shahi, V.K. A Facile Synthesis of Highly Stable Multiblock Poly(Arylene Ether)s Based Alkaline Membranes for Fuel Cells. J. Power Sources 2014, 267, 714–722. [Google Scholar] [CrossRef]
- Li, X.; Nie, G.; Tao, J.; Wu, W.; Wang, L.; Liao, S. Assessing the Influence of Side-Chain and Main-Chain Aromatic Benzyltrimethyl Ammonium on Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2014, 6, 7585–7595. [Google Scholar] [CrossRef]
- Yokota, N.; Ono, H.; Miyake, J.; Nishino, E.; Asazawa, K.; Watanabe, M.; Miyatake, K. Anion Conductive Aromatic Block Copolymers Containing Diphenyl Ether or Sulfide Groups for Application to Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 17044–17052. [Google Scholar] [CrossRef]
- Inaba, M.; Matsui, Y.; Saito, M.; Tasaka, A.; Fukuta, K.; Watanabe, S.; Yanagi, H. Effects of Carbon Dioxide on the Performance of Anion-Exchange Membrane Fuel Cells. Electrochemistry 2011, 79, 322–325. [Google Scholar] [CrossRef]
- Yan, J.; Hickner, M.A. Anion Exchange Membranes by Bromination of Benzylmethyl-Containing Poly(Sulfone)s. Macromolecules 2010, 43, 2349–2356. [Google Scholar] [CrossRef]
- Tanaka, M.; Koike, M.; Miyatake, K.; Watanabe, M. Anion Conductive Aromatic Ionomers Containing Fluorenyl Groups. Macromolecules 2010, 43, 2657–2659. [Google Scholar] [CrossRef]
- Shimada, M.; Shimada, S.; Miyake, J.; Uchida, M.; Miyatake, K. Anion Conductive Aromatic Polymers Containing Fluorenyl groups: Effect of the Position and Number of Ammonium Groups. J. Polym. Sci. Part A Polym. Chem. 2015, 54, 935–944. [Google Scholar] [CrossRef]
- Zhu, X.; Tokash, J.; Hong, Y.; Logan, B.E. Controlling the Occurrence of Power Overshoot by Adapting Microbial Fuel Cells to High Anode Potentials. Bioelectrochemistry 2013, 90, 30–35. [Google Scholar] [CrossRef]











| Polymers | Mntarget (Da) /DP | MnNMR (Da) /DP | MnGPC (Da) /DP | PDI a |
|---|---|---|---|---|
| PES-1 | 4000/8 | 5140/10 | 5110/10 | 1.67 |
| PES-2 | 8000/13 | 8680/14 | 8060/13 | 1.55 |
| PES-3 | - | - | 8480 | 1.65 |
| Cardo-PES | - | - | 27,200 | 4.34 |
| Bromination Yield a (%) | DBM b (%) | IECW (mequiv./g) | |||
|---|---|---|---|---|---|
| Cardo-PES-QA | Cardo-PES-IM | ||||
| Theo. c | Titr. d | Theo. c | Titr. d | ||
| 72.9 | 72.9 | 1.54 | 1.49 | 1.42 | 1.38 |
| Polymer | IECW,theo (mequiv./g) | WUW (%) | λ | Δl (%) | Δt (%) | σ (mS/cm) | Ea a (KJ/mol) | ||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 80 °C | 20 °C | 20 °C | 20 °C | 20 °C | 80 °C | |||
| Cardo-PES-QA | 1.54 | 80 | 110 | 28.8 | 16.8 | 25.2 | 38 | 86 | 13.46 |
| Cardo-PES-IM | 1.42 | 69 | 92 | 26.9 | 12 | 16.7 | 30 | 45 | 6.23 |
| Membrane | Ionic Group | IEC (mequiv./g) | Conductivity (mS/cm) |
|---|---|---|---|
| Cardo-PES-QA [This work] | QA | 38 | 38 at 20 °C |
| Cardo-PES-IM [This work] | IM | 30 | 30 at 20 °C |
| QPE-X16Y11 [14] | QA | 1.38 | 52 at 60 °C |
| PSf80-ImOH [26] | IM | 1.39 | 16.1 at 20 °C |
| QRPES-40 [35] | QA | 1.50 | 21 at room temperature |
| IM-PFEKS [23] | IM | 1.64 | 17.1 at room temperature |
| ATMPP [27] | QA | 1.57 | 50 at 30 °C |
| PSF-PTMA(X62) [36] | QA | 146 | 33 at 20 °C |
| QPAE-d [38] | QA | 1.43 | 5.8 at 20 °C |
| PSf-ImOH 90% [28] | IM | 1.58 | ~20 at 20 °C |
| QPAE-X25Y21 [39] | QA | 1.45 | 16.9 at 20 °C |
| NAPEK-Q-100 [40] | QA | 1.46 | 23 at 20 °C |
| mPES-X9.2Y3.4 [41] | QA | 1.45 | 4.9 at 25 °C |
| EI-PES [42] | IM | 1.45 | 30 at 20 °C |
| bQAPDHTPE-OH20 [29] | QA | 1.66 | 21.37 at 30 °C |
| AMPE-M15N15 [43] | QA | 1.33 | 34.4 at 30 °C |
| QPAES-X16Y10 [30] | QA | 1.45 | 15.4 at 20 °C |
| PAES-Q-75 [44] | QA | 1.49 | 21.9 at 25 °C |
| QPE-bl-5 (X9Y8) [45] | QA | 1.5 | ~41 at 60 °C |
| ImPESN-9–22 [31] | IM | 1.58 | 38.5 at 30 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, A.K.; Song, Y.E.; Kim, J.R.; Kim, N.; Paik, H.-j. Phenolphthalein Anilide Based Poly(Ether Sulfone) Block Copolymers Containing Quaternary Ammonium and Imidazolium Cations: Anion Exchange Membrane Materials for Microbial Fuel Cell. Membranes 2021, 11, 454. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11060454
Mohanty AK, Song YE, Kim JR, Kim N, Paik H-j. Phenolphthalein Anilide Based Poly(Ether Sulfone) Block Copolymers Containing Quaternary Ammonium and Imidazolium Cations: Anion Exchange Membrane Materials for Microbial Fuel Cell. Membranes. 2021; 11(6):454. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11060454
Chicago/Turabian StyleMohanty, Aruna Kumar, Young Eun Song, Jung Rae Kim, Nowon Kim, and Hyun-jong Paik. 2021. "Phenolphthalein Anilide Based Poly(Ether Sulfone) Block Copolymers Containing Quaternary Ammonium and Imidazolium Cations: Anion Exchange Membrane Materials for Microbial Fuel Cell" Membranes 11, no. 6: 454. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11060454