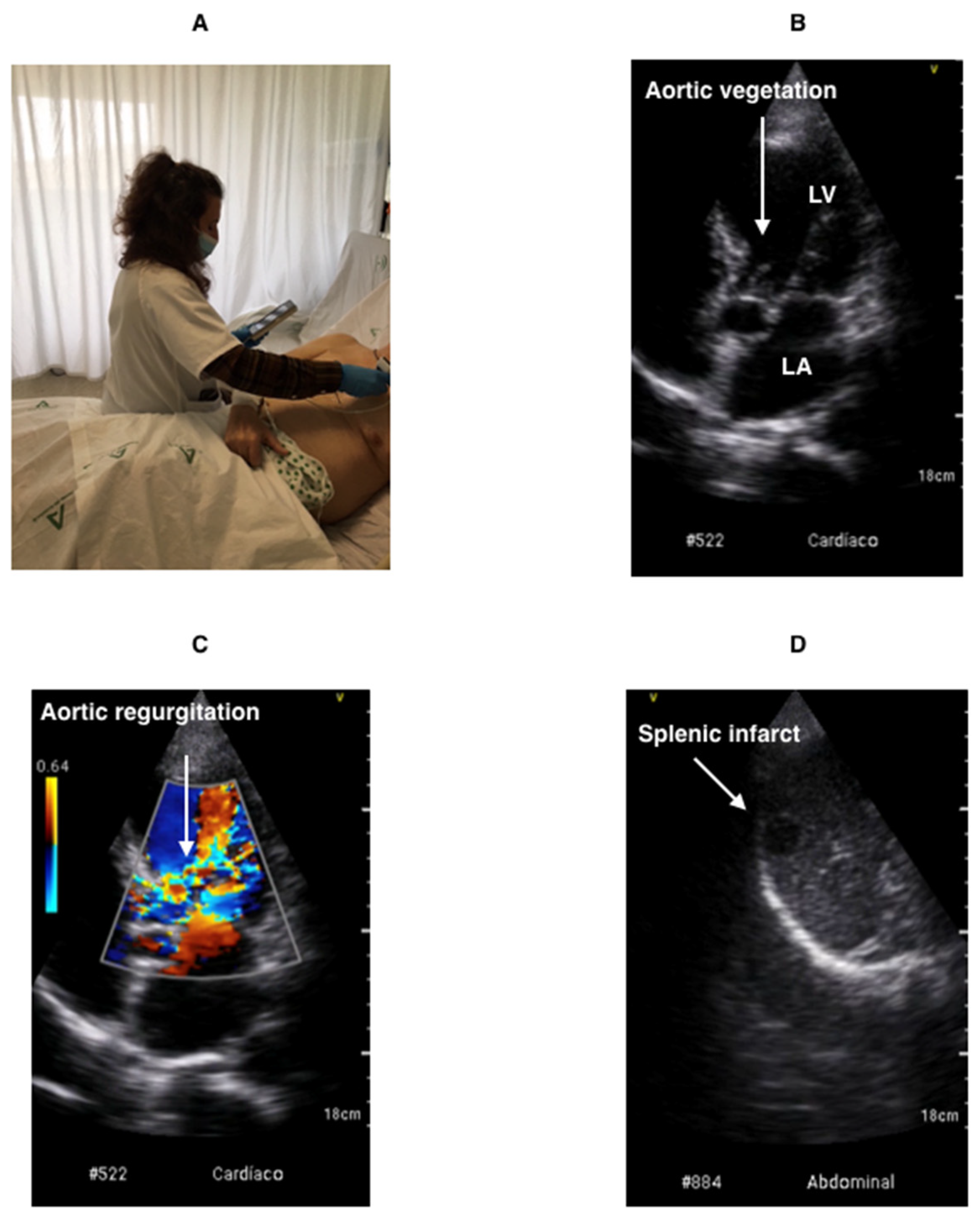
Point-of-Care Ultrasound (POCUS) as an Extension of the Physical Examination in Patients with Bacteremia or Candidemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Sample
2.3. Epidemiological, Clinical, Laboratory and Ultrasound Data Collection
2.4. Outcome Measures and Definitions
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Hepatomegaly. 0. No 1. Yes Hepatic infarction. 0. No 1. Yes Renal infarction. 0. No 1. Yes | Splenomegaly. 0. No 1. Yes Splenic infarction. 0. No 1. Yes Ascites. 0. No 1. Yes |
References
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef] [Green Version]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hidalgo, N.; Almirante, B. La endocarditis infecciosa en el siglo xxi: Cambios epidemiológicos, terapéuticos y pronósticos. Enferm. Infecc. Microbiol. Clin. 2012, 30, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The task force of the management of infective endocarditis of the European Society of Cardiology. Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.L.; Arnold, C.J.; Fowler, V.G. Clinical Management of Staphylococcus aureus Bacteremia: A review. JAMA 2014, 312, 1330–1341. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, R.V.; Høst, U.; Arpi, M.; Hassager, C.; Johansen, H.K.; Korup, E.; Schønheyder, H.C.; Berning, J.; Gill, S.; Rosenvinge, F.S.; et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: The value of screening with echocardiography. Eur. J. Echocardiogr. 2011, 12, 414–420. [Google Scholar] [CrossRef]
- Bai, A.; Steinberg, M.; Showler, A.; Burry, L.; Bhatia, R.S.; Tomlinson, G.; Bell, C.M.; Morris, A.M. Diagnostic Accuracy of Transthoracic Echocardiography for Infective Endocarditis Findings Using Transesophageal Echocardiography as the Reference Standard: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 639–646.e8. [Google Scholar] [CrossRef]
- Sivak, J.A.; Vora, A.N.; Navar, A.M.; Schulte, P.J.; Crowley, A.L.; Kisslo, J.; Corey, G.R.; Liao, L.; Wang, A.; Velazquez, E.J.; et al. An Approach to Improve the Negative Predictive Value and Clinical Utility of Transthoracic Echocardiography in Suspected Native Valve Infective Endocarditis. J. Am. Soc. Echocardiogr. 2016, 29, 315–322. [Google Scholar] [CrossRef]
- Yuan, X.-C.; Liu, M.; Hu, J.; Zeng, X.; Zhou, A.-Y.; Chen, L. Diagnosis of infective endocarditis using echocardiography. Medicine 2019, 98, e17141. [Google Scholar] [CrossRef]
- Showler, A.; Burry, L.; Bai, A.D.; Steinberg, M.; Ricciuto, D.R.; Fernandes, T.; Chiu, A.; Raybardhan, S.; Science, M.; Fernando, E.; et al. Use of transthoracic echocardiography in the management of low-risk Staphylococcus aureus bacteremia: Results from a retrospective multicenter cohort study. JACC Cardiovasc. Imag. 2015, 8, 924. [Google Scholar] [CrossRef] [Green Version]
- Abu Saleh, O.; Fida, M.; Asbury, K.; Narichania, A.; Sotello, D.; Bosch, W.; Vikram, H.R.; Palraj, R.; Lahr, B.; Baddour, L.M.; et al. Prospective Validation of PREDICT and Its Impact on the Transesophageal Echocardiography Use in Management of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2020, 73, e1745–e1753. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.L.; Copel, J.A. Point-of-Care Ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Via, G.; Hussain, A.; Wells, M.; Reardon, R.; Elbarbary, M.; Noble, V.E.; Tsung, J.; Neskovic, A.; Price, S.; Oren-Grinberg, A.; et al. International Evidence-Based Recommendations for Focused Cardiac Ultrasound. J. Am. Soc. Echocardiogr. 2014, 27, 683.e1–683.e33. [Google Scholar] [CrossRef] [PubMed]
- De Isla, L.P.; Sánchez, S.D.; Pagola, J.; Sánchez, G.G.D.C.; Fernández, T.L.; Barrancos, I.M.S.; Martínez-Sánchez, P.; Gaviria, A.Z.; Anguita, M.; Serrano, A.L.R.; et al. Consensus Document of the SEMI, semFYC, SEN, and SEC on Focused Cardiac Ultrasound in Spain. Rev. Esp. Cardiol. (Engl. Ed.) 2018, 71, 935–940. [Google Scholar] [CrossRef]
- Torres Macho, J.; García Sánchez, F.J.; Garmilla Ezquerra, P.; Romero, L.B.; Lebrato, J.C.; Rojo, J.C.; Arribase, A.; Pintos, P.; Martínez, P.; Rodrigo, C.; et al. Positioning document on incorporating point-of-care ultrasound in Internal Medicine departments. Rev. Clin. Esp. (Engl. Ed.) 2018, 218, 192–198. [Google Scholar] [CrossRef]
- Marbach, J.A.; Almufleh, A.; Di Santo, P.; Jung, R.; Simard, T.; McInnes, M.; Salameh, J.-P.; McGrath, T.A.; Millington, S.J.; Diemer, G.; et al. Comparative Accuracy of Focused Cardiac Ultrasonography and Clinical Examination for Left Ventricular Dysfunction and Valvular Heart Disease. Ann. Intern. Med. 2019, 171, 264. [Google Scholar] [CrossRef]
- Kobal, S.L.; Liel-Cohen, N.; Shimony, S.; Neuman, Y.; Konstantino, Y.; Dray, E.M.; Horowitz, I.; Siegel, R.J. Impact of Point-of-Care Ultrasound Examination on Triage of Patients With Suspected Cardiac Disease. Am. J. Cardiol. 2016, 118, 1583–1587. [Google Scholar] [CrossRef]
- Chamsi-Pasha, M.A.; Sengupta, P.P.; Zoghbi, W.A. Handheld Echocardiography. Circulation 2017, 136, 2178–2188. [Google Scholar] [CrossRef]
- Narula, J.; Chandrashekhar, Y.; Braunwald, E. Time to Add a Fifth Pillar to Bedside Physical Examination. JAMA Cardiol. 2018, 3, 346–350. [Google Scholar] [CrossRef]
- Jenkins, S.; Alabed, S.; Swift, A.; Marques, G.; Ryding, A.; Sawh, C.; Wardley, J.; Shah, B.N.; Swoboda, P.; Senior, R.; et al. Diagnostic accuracy of handheld cardiac ultrasound device for assessment of left ventricular structure and function: Systematic review and meta-analysis. Heart 2021, 107, 1826–1834. [Google Scholar] [CrossRef]
- Bonzi, M.; Cernuschi, G.; Solbiati, M.; Giusti, G.; Montano, N.; Ceriani, E. Diagnostic accuracy of transthoracic echocardiography to identify native valve infective endocarditis: A systematic review and meta-analysis. Intern. Emerg. Med. 2018, 13, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Casado-López, I.; Tung-Chen, Y.; Torres-Arrese, M.; Luordo-Tedesco, D.; Mata-Martínez, A.; Casas-Rojo, J.M.; Montero-Hernández, E.; De Casasola-Sánchez, G.G. Usefulness of Multi-Organ Point-of-Care Ultrasound as a Complement to the Decision-Making Process in Internal Medicine. J. Clin. Med. 2022, 11, 2256. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.T.; Flachskampf, F.A. Focused Cardiac Ultrasonography. JACC Cardiovasc. Imag. 2019, 12, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Scali, M.C.; Ciampi, Q.; Lichtenstein, D. Lung Ultrasound for the Cardiologist. JACC Cardiovasc. Imag. 2018, 11, 1692–1705. [Google Scholar] [CrossRef]
- Zúñiga, M.L.; Palomino, T.V.; Toro, M.A.M.; Fernández, A.M.C.; Neira, D.G.; Parras, A.M.V.; García, M.I.V.; Colmenero, J.M.; Moreno, F.P.; Calero, A.C.; et al. Diagnostic Capacity of Pocket-Sized Ultrasound Devices at Point of Care by a Non-radiologist Resident in Patients with Suspected Abdominal Pathology. Ultrasound Med. Biol. 2019, 46, 263–268. [Google Scholar] [CrossRef]
- Marbach, J.A.; Almufleh, A.; Di Santo, P.; Simard, T.; Jung, R.; Diemer, G.; West, F.M.; Millington, S.J.; Mathew, R.; Le May, M.R.; et al. A shifting paradigm: The role of focused cardiac ultrasound in bedside patient assessment. Chest 2020, 158, 2107–2118. [Google Scholar] [CrossRef]
- De La Vega, P.B.; Tandon, P.; Qureshi, W.; Nasr, Y.; Jayaprakash, R.; Arshad, S.; Moreno, D.; Jacobsen, G.; Ananthasubramaniam, K.; Ramesh, M.; et al. Simplified risk stratification criteria for identification of patients with MRSA bacteremia at low risk of infective endocarditis: Implications for avoiding routine transesophageal echocardiography in MRSA bacteremia. Eur. J. Clin. Microbiol. 2015, 35, 261–268. [Google Scholar] [CrossRef]
| Staphylococcus aureus |
| Viridans streptococcus: S. mitis, S. sanguis, S. mutans, S. milleri, S. salivarius |
| Streptococcus gallolyticus (formerly, S. bovis) |
| HACEK group: Haemophilus aphrophilus (subsequently called Aggregatibacter aphrophilus), Actinobacillus actinomycetemcomitans (subsequently called Aggregatibacter actinomycetemcomitans), Cardiobacterium hominis, Eikenella corrodens, Kingella kingae. |
| Enterococcus spp. |
| Microorganisms usually considered epithelial contaminants, isolation in three or four blood culture flasks: Coagulase-negative staphylococcus, Corynebacterium spp., Cutybacterium acnes, Bacillus spp. |
| Candida spp. |
| Others: Pseudomonas aeruginosa, Acinetobacter spp., Coxiella burnetti (IgG phase I > 1:800), Brucella spp., Bartonella spp., Chlamydia psittaci, Legionella spp., Mycoplasma spp., Tropheryma whippelii, Lactobacillus spp., Gordonia bronchialis, Erysipelothrix rhusiopathiae, Neisseria elongata, Moraxella catarrhalis, Veillonella spp., Listeria monocytogenes, Campylobacter fetus, Francisella tularensis, Catabacter hongkongensi |
| Patients n: 258 | H.U.T. | H.U.J | H.U.To. | H.U.MS | H.U.VB | H.U.V.N. | p |
|---|---|---|---|---|---|---|---|
| Patients recruited, n (%) | 101 (39.2%) | 54 (20.9%) | 45 (17.4%) | 38 (14.7%) | 12 (4.7%) | 8 (3.8%) | 0.001 * |
| Age, years (mean) | 65.1 | 67.8 | 71.1 | 67.7 | 66.7 | 70.8 | 0.776 ** |
| Gender (female), n (%) | 38 (37.6%) | 20 (37%) | 12 (26.6%) | 17 (44.7%) | 4 (33.3%) | 3 (37.5%) | 0.683 * |
| Number of POCUS examinations/year performed by involved operators | 500 | 450 | 220 | 250 | 350 | 150 | <0.001 * |
| Clinical manifestations, n (%) | |||||||
| Fever | 93 (92.1%) | 53 (98.14%) | 45 (100%) | 38 (100%) | 12 (100%) | 8 (100%) | 0.074 * |
| Duration of the fever, days (mean) | 2.8 | 1.8 | 1.9 | 1.7 | 2.6 | 2.5 | 0.182 ** |
| Shivering | 64 (63.3%) | 36 (66.6%) | 32 (71.1%) | 14 (36.8%) | 7 (58.3%) | 6 (75%) | 0.024 * |
| Anorexia | 65 (64.3%) | 29 (53.7%) | 31 (68.8%) | 15 (39.5%) | 4 (%) | 4 (50%) | 0.026 * |
| Weight loss | 32 (31.7%) | 20 (37%) | 18 (40%) | 5 (13.1%) | 3 (33.3%) | 1 (12.5%) | 0.080 * |
| Dyspnoea | 31 (30.7%) | 22 (40.7%) | 17 (33.7%) | 11 (28.9%) | 6 (50%) | 3 (37.5%) | 0.611 * |
| Myalgia | 36 (35.6%) | 17 (31.5%) | 11 (22.4%) | 3 (7.9%) | 5 (41.6%) | 2 (25%) | 0.034 * |
| Night sweats | 34 (33.6%) | 16 (29.6%) | 14 (31.1%) | 4 (10.5%) | 3 (25%) | 3 (37.5%) | 0.163 * |
| Heart murmur | 33 (32.6%) | 13 (24%) | 16 (35.5%) | 10 (26.3%) | 5 (41.6%) | 3 (37.5%) | 0.705 * |
| Risk factors, n (%) | |||||||
| Prosthetic heart valve | 3 (2.9%) | 11 (20%) | 10 (22%) | 3 (7.9%) | 3 (25%) | 1 (12.5%) | 0.003 * |
| Congenital heart disease | 0 | 3 (5.5%) | 1 (2.2%) | 0 | 0 | 0 | 0.140 * |
| Permanent pacemaker | 8 (7.9%) | 3 (5.5%) | 9 (20%) | 1 (2.6%) | 2 (16.6%) | 0 | 0.048 * |
| ICD | 2 (1.9%) | 2 (3.7%) | 1 (2.2%) | 2 (5.2%) | 0 | 0 | 0.857 * |
| Charlson Index (mean) | 3.5 | 4.2 | 4.7 | 2.9 | 4.1 | 4.1 | 0.014 ** |
| Deficient oral hygiene | 49 (48.5%) | 11 (20.3%) | 23 (51.1%) | 4 (10.5%) | 6 (50%) | 1 (12.5%) | 0.001 * |
| Dental procedures | 2 (1.9%) | 2 (3.7%) | 3 (6.6%) | 2 (5.3%) | 0 | 0 | 0.682 * |
| Endoscopy | 14 (13.8%) | 4 (7.4%) | 4 (8.8%) | 4 (10.5%) | 1 (8.3%) | 1 (12.5%) | 0.864 * |
| Surgery | 24 (23.7%) | 6 (11.1%) | 2 (4.4%) | 5 (13.2%) | 1 (8.3%) | 1 (12.5%) | 0.049 * |
| Immunosuppression | 24 (23.8%) | 11 (20.4%) | 9 (20%) | 15 (39.5%) | 3 (25%) | 2 (25%) | 0.350 * |
| Diabetes mellitus | 39 (38.6%) | 17 (31.5%) | 18 (40%) | 12 (31.6%) | 4 (33.3%) | 5 (62.5%) | 0.582 * |
| Chronic kidney disease | 29 (28.7%) | 14 (25.9%) | 5 (11.1%) | 4 (10.5%) | 4 (33.3%) | 2 (25%) | 0.078 * |
| Microbiology, n (%) | |||||||
| MSSA | 25 (24.7%) | 15 (27.7%) | 12 (26.6%) | 7 (18.4%) | 3 (25%) | 2 (25%) | 0.948 * |
| MRSA | 5 (4.9%) | 2 (3.7%) | 5 (11.1%) | 9 (23.7%) | 2 (16.6%) | 1 (12.5%) | 0.012 * |
| CNS | 16 (5.9%) | 8 (14.8%) | 5 (11.1%) | 4 (10.5%) | 0 | 0 | 0.512 * |
| Viridans streptococci | 6 (5.9%) | 1 (1.8%) | 4 (8.8%) | 4 (10.5%) | 3 (25%) | 2 (25%) | 0.036 * |
| Other streptococci | 14 (13.8%) | 8 (14.8%) | 6 (13.3%) | 2 (5.3%) | 4 (33.3%) | 0 | 0.172 * |
| Enterococci spp. | 14 (13.8%) | 14 (25.9%) | 9 (20%) | 2 (5.3%) | 0 | 0 | 0.031 * |
| Candida spp. | 19 (18.8%) | 3 (5.5%) | 1 (2.2%) | 8 (21.1%) | 0 | 2 (25%) | 0.009 * |
| Other microorganisms | 2 (1.9%) | 3 (5.5%) | 3 (6.6%) | 2 (5.3%) | 0 | 1 (12.5%) | 0.534 * |
| Duration of POCUS examination and interpretation, minutes (mean) | 9:43 | 08:26 | 23:39 | 15:36 | 15:02 | 11:03 | 0.001 ** |
| Definite (n) | Possible (n) | Rejected (n) | |
|---|---|---|---|
| MSSA | 13 | 2 | 49 |
| MRSA | 3 | 2 | 19 |
| CNS | 10 | 1 | 22 |
| Viridans streptococcus | 8 | 1 | 11 |
| Other streptococcus | 11 | 1 | 22 |
| Enterococcus spp. | 13 | 1 | 25 |
| Candida spp. | 0 | 1 | 32 |
| Other microorganisms | 6 | 9 | 5 |
| Total, n (%) | 64 (24.8%) | 9 (3.5%) | 185 (71.7%) |
| Patients (n) | 73 |
|---|---|
| Location, n (%) | |
| Aortic | 33 (45.2%) |
| Mitral | 30 (41.1%) |
| Tricuspid | 5 (6.8%) |
| Aortic + mitral | 2 (2.7%) |
| Pacemaker lead/ICD | 3 (4.1%) |
| Type of valve, n (%) | |
| Native | 50 (68.5%) |
| Prosthetic | 20 (27.4%) |
| Pacemaker lead/ICD | 3 (4.1%) |
| Acquisition, n (%) | |
| Community | 45 (61.6%) |
| Healthcare-related | 28 (38.4%) |
| Heart surgery, n (%) | |
| Scheduled | 16 (76.2%) |
| Urgent | 5 (23.8%) |
| Indication for heart surgery, n (%) | |
| Heart failure | 7 (33.3%) |
| Uncontrolled infection | 11 (5.,4%) |
| Prevent septic embolism | 3 (14.3%) |
| TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Concordance (Kappa) | |
|---|---|---|---|---|---|---|---|---|---|
| Valvular vegetation | 52 | 11 | 15 | 180 | 0.77 | 0.94 | 0.82 | 0.92 | 0.733 |
| (0.67–0.87) | (0.90–0.97) | (0.61–0.92) | (0.88–0.96) | ||||||
| Aortic valve vegetation | 17 | 4 | 11 | 226 | 0.61 | 0.98 | 0.81 | 0.95 | 0.662 |
| (0.43–0.79) | (0.97–0.99) | (0.61–0.99) | (0.92–0.98) | ||||||
| Mitral valve vegetation | 30 | 5 | 5 | 218 | 0.86 | 0.97 | 0.86 | 0.97 | 0.835 |
| (0.74–0.97) | (0.96–0.99) | (0.72–0.98) | (0.95–0.99) | ||||||
| Tricuspid valve vegetation | 5 | 3 | 5 | 245 | 0.5 | 0.99 | 0.62 | 0.98 | 0.540 |
| (0.19–0.81) | (0.97–1) | (0.22–0.99) | (0.96–0.99) | ||||||
| Aortic regurgitation * | 67 | 20 | 21 | 150 | 0.76 | 0.88 | 0.77 | 0.87 | 0.645 |
| (0.67–0.85) | (0.83–0.93) | (0.67–0.86) | (0.82–0.92) | ||||||
| Mitral regurgitation * | 91 | 20 | 28 | 119 | 0.76 | 0.85 | 0.82 | 0.81 | 0.624 |
| (0.69–0.84) | (0.79–0.91) | (0.74–0.89) | (0.74–0.87) | ||||||
| Tricuspid regurgitation * | 81 | 18 | 23 | 136 | 0.77 | 0.88 | 0.82 | 0.85 | 0.667 |
| (0.69–0.85) | (0.83–0.93) | (0.73–0.89) | (0.79–0.91) | ||||||
| LV systolic dysfunction | 22 | 5 | 5 | 226 | 0.81 | 0.98 | 0.81 | 0.98 | 0.793 |
| (0.66–0.96) | (0.95–0.99) | (0.65–0.98) | (0.96–0.99) | ||||||
| LV dilatation | 15 | 3 | 4 | 236 | 0.79 | 0.98 | 0.83 | 0.98 | 0.796 |
| (0.60–0.97) | (0.97–1) | (0.63–0.99) | (0.96–0.99) | ||||||
| LA dilatation | 92 | 14 | 18 | 134 | 0.84 | 0.90 | 0.86 | 0.88 | 0.745 |
| (0.76–0.90) | (0.85–0.95) | (0.80–0.94) | (0.83–0.94) | ||||||
| RA dilatation | 37 | 11 | 10 | 200 | 0.78 | 0.95 | 0.77 | 0.95 | 0.729 |
| (0.67–0.90) | (0.91–0.97) | (0.64–0.90) | (0.92–0.98) | ||||||
| RV dilatation | 17 | 8 | 4 | 229 | 0.81 | 0.96 | 0.68 | 0.98 | 0.714 |
| (0.64–0.97) | (0.97–0.99) | (0.47–0.88) | (0.96–0.99) | ||||||
| Pericardial effusion | 14 | 9 | 2 | 233 | 0.87 | 0.96 | 0.61 | 0.99 | 0.696 |
| (0.71–1) | (0.94–0.99) | (0.39–0.83) | (0.98–0.99) |
| TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Concordance (Kappa) | |
|---|---|---|---|---|---|---|---|---|---|
| Hepatomegaly | 12 | 7 | 1 | 166 | 0.92 | 0.96 | 0.63 | 0.99 | 0.727 |
| (0.77–1) | (0.93–0.99) | (0.39–0.87) | (0.98–1) | ||||||
| Splenomegaly | 11 | 3 | 1 | 171 | 0.92 | 0.98 | 0.78 | 0.99 | 0.935 |
| (0.76–1) | (0.96–1) | (0.53–1) | (0.98–1) | ||||||
| Hepatic infarction | 3 | 1 | 2 | 180 | 0.6 | 0.99 | 0.75 | 0.99 | 0.659 |
| (0.17–1) | (0.98–1) | (0.20–0.99) | (0.97–1) | ||||||
| Splenic infarction | 4 | 0 | 4 | 178 | 0.5 | 1 | 1 | 0.98 | 0.657 |
| (0.15–0.84) | (1–1) | (0.87–1) | (0.95–1) | ||||||
| Renal infarction | 0 | 1 | 5 | 180 | - | 0.99 | - | 0.97 | - |
| (0.98–1) | (0.94–0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Palmero, S.; López Zúñiga, M.A.; Rodríguez Martínez, V.; Reyes Parrilla, R.; Alguacil Muñoz, A.M.; Sánchez-Yebra Romera, W.; Martín Rico, P.; Poquet Catalá, I.; Jiménez Guardiola, C.; Del Pozo Pérez, A.; et al. Point-of-Care Ultrasound (POCUS) as an Extension of the Physical Examination in Patients with Bacteremia or Candidemia. J. Clin. Med. 2022, 11, 3636. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm11133636
López Palmero S, López Zúñiga MA, Rodríguez Martínez V, Reyes Parrilla R, Alguacil Muñoz AM, Sánchez-Yebra Romera W, Martín Rico P, Poquet Catalá I, Jiménez Guardiola C, Del Pozo Pérez A, et al. Point-of-Care Ultrasound (POCUS) as an Extension of the Physical Examination in Patients with Bacteremia or Candidemia. Journal of Clinical Medicine. 2022; 11(13):3636. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm11133636
Chicago/Turabian StyleLópez Palmero, Serafín, Miguel Angel López Zúñiga, Virginia Rodríguez Martínez, Raul Reyes Parrilla, Ana Maria Alguacil Muñoz, Waldo Sánchez-Yebra Romera, Patricia Martín Rico, Inmaculada Poquet Catalá, Carlos Jiménez Guardiola, Alfonso Del Pozo Pérez, and et al. 2022. "Point-of-Care Ultrasound (POCUS) as an Extension of the Physical Examination in Patients with Bacteremia or Candidemia" Journal of Clinical Medicine 11, no. 13: 3636. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm11133636






