Outcomes of Social Egg Freezing: A Cohort Study and a Comprehensive Literature Review
Abstract
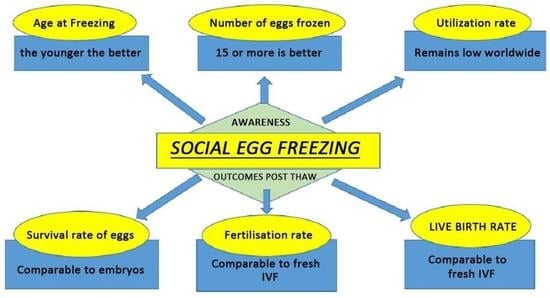
:1. Introduction
2. Materials and Methods
2.1. Local Data Collection
2.2. Data and Statistical Analysis
2.3. Treatment Protocol
2.4. Literature Review
2.5. Study Selection Process
3. Results
3.1. Outcomes of Thaw Cycles
3.2. Results from Literature Review
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teo, U.L.; Kakkar, P.; El-Toukhy, T. Current Perspectives on Social Oocyte Freezing. J. Obstet. Gynaecol. 2021, 42, 370–378. [Google Scholar] [CrossRef]
- ASRM. Mature oocyte cryopreservation: A guideline. American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Fertil. Steril. 2013, 99, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Richings, N.M.; Leung, A.; Sakkas, D.; Catt, S. A major increase in oocyte cryopreservation cycles in the USA, Australia and New Zealand since 2010 is highlighted by younger women but a need for standardized data collection. Hum. Reprod. 2021, 36, 624–635. [Google Scholar] [CrossRef]
- Peate, M.; Sandhu, S.; Braat, S.; Hart, R.; Norman, R.; Parle, A.; Lew, R.; Hickey, M. Randomized control trial of a decision aid for women considering elective egg freezing: The Eggsurance study protocol. Womens Health 2022, 18, 17455057221139673. [Google Scholar] [CrossRef] [PubMed]
- Potdar, N.; Gelbaya, T.A.; Nardo, L.G. Oocyte vitrification in the 21st century and post-warming fertility outcomes: A systematic review and meta-analysis. Reprod. Biomed. Online 2014, 29, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Wise, J. UK lifts ban on frozen eggs. BMJ 2000, 320, 334. [Google Scholar]
- ASRM. Planned oocyte cryopreservation for women seeking to preserve future Reproductive potential: An Ethics Committee opinion. Fertil. Steril. 2018, 110, 1022–1028. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, E.A.; Pasch, L.A.; Hastie, J.; Cedars, M.I.; Huddleston, H.G. To freeze or not to Freeze: Decision regret and satisfaction following elective oocyte cryopreservation. Fertil. Steril. 2018, 109, 1097–1104. [Google Scholar] [CrossRef]
- Seyhan, A.; Akin, O.D.; Ertaş, S.; Ata, B.; Yakin, K.; Urman, B. A Survey of Women Who Cryopreserved Oocytes for Non-medical Indications (Social Fertility Preservation). Reprod Sci. 2021, 28, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.; Culley, L. Women’s Experience of Social Egg Freezing: Perceptions of Success, Risks, and ‘Going It Alone’. Hum. Fertil. 2018, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Drost, L.; Dason, E.S.; Han, J.; Doshi, T.; Scheer, A.; Greenblatt, E.M.; Jones, C.A. Patients’ and providers’ perspectives on non-urgent egg freezing decision-making: A thematic analysis. BMC Womens Health 2023, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.; Culley, L.; Hudson, N.; Mitchell, H.; Lavery, S. Oocyte cryopreservation for social reasons: Demographic profile and disposal intentions of UK users. Reprod. Biomed. Online 2015, 31, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ESHRE Task Force on Ethics and Law. Oocyte cryopreservation for age-related fertility loss. Human. Reprod. 2012, 27, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Kasaven, L.S.; Jones, B.P.; Heath, C.; Odia, R.; Green, J.; Petrie, A.; Saso, S.; Serhal, P.; Ben Nagi, J. Reproductive outcomes from ten years of elective oocyte cryopreservation. Arch. Gynecol. Obstet. 2022, 306, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Tsafrir, A.; Ben-Ami, I.; Eldar-Geva, T.; Gal, M.; Dekel, N.; Levi, H.; Schonberger, O.; Srebnik, N.; Weintraub, A.; Goldberg, D.; et al. Clinical outcome of planned oocyte cryopreservation at advanced age. J. Assist. Reprod. Genet. 2022, 39, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.-J.; Wu, M.-Y.; Chao, K.-H.; Wei, S.-Y.; Tsai, Y.-Y.; Huang, T.-C.; Chen, M.-J.; Chen, S.-U. Usage and cost-effectiveness of elective oocyte freezing: A retrospective observational study. Reprod. Biol. Endocrinol. 2022, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, S.K.; Coomarasamy, A.; Khalaf, Y.; Braude, P. A three-arm randomised controlled trial comparing GnRH agonist long regimen versus GnRH antagonist short regimen in women with a history of poor ovarian response undergoing in vitro fertilisation treatment. Poor responders intervention trial (PRINT). Reprod. Health 2007, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- El-Tokhy, O.; Kopeika, J.; El-Toukhy, T. An update on prevention of ovarian hyperstimulatiopnsyndrome. Womens Health 2016, 12, 496–503. [Google Scholar]
- VerMilyea, M.; Brewer, A. Appendix D: Irvine Scientific Vitrification system. Methods Mol. Biol. 2017, 44, 1271–1278. [Google Scholar]
- Esfandiari, N.; Litzky, J.; Sayler, J.; Zagadailov, P.; George, K.; Demars, L. Egg freezing for fertility preservation and family planning: A nationwide survey of US Obstetrics and Gynecology residents. Reprod. Biol. Endocrinol. 2019, 17, 16. [Google Scholar] [CrossRef] [Green Version]
- Harjee, R.; Chen, J.; Caudle, J.; Ouhibi, N.; Edsall, S.; Smrz, J.; Lardizabal, J.; Abdelghadir, S.; Nakhuda, G. Oocyte Cryopreservation: A 9-Year Single-Centre Experience. J. Obs. Gynaecol. Can. 2022, 44, 1271–1278. [Google Scholar] [CrossRef]
- Blakemore, J.K.; Grifo, J.A.; DeVore, S.M.; Hodes-Wertz, B.; Berkeley, A.S. Planned oocyte cryopreservation-10-15-year follow-up: Return rates and cycle outcomes. Fertil. Steril. 2021, 115, 1511–1520. [Google Scholar] [CrossRef]
- Gürtin, Z.B.; Morgan, L.; O’rourke, D.; Wang, J.; Ahuja, K. For whom the egg thaws: Insights from an analysis of 10 years of frozen egg thaw data from two UK clinics, 2008–2017. J. Assist. Reprod. Genet. 2019, 36, 1069–1108. [Google Scholar] [CrossRef]
- Wafi, A.; Nekkebroeck, J.; Blockeel, C.; De Munck, N.; Tournaye, H.; De Vos, M. A follow-up survey on the reproductive intentions and experiences of women undergoing planned oocyte cryopreservation. Reprod. Biomed. Online 2020, 40, 207–214. [Google Scholar] [CrossRef]
- Cobo, A.; García-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and Onco-fertility preservation: Factors related to IVF outcomes. Hum. Reprod. 2018, 33, 2222–2223. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.L.; Schildauer, K.; Brännström, M. Elective oocyte freezing for nonmedical reasons: A 6-year report on utilization and in vitro fertilization results from a Swedish center. Acta Obstet. Et. Gynecol. Scand. 2018, 98, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.P.; Anderson, R.E.; Feinberg, E.C.; Hayward, B.; Mahony, M.C. The Human Oocyte Preservation Experience (HOPE) Registry: Evaluation of cryopreservation techniques and oocyte source on outcomes. Reprod. Biol. Endocrinol. 2017, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Mesen, T.B.; Mersereau, J.E.; Kane, J.B.; Steiner, A.Z. Optimal timing for elective egg freezing. Fertil. Steril. 2015, 103, 1551–1556.e4. [Google Scholar] [CrossRef] [Green Version]
- Walker, Z.; Lanes, A.; Ginsburg, E. Oocyte cryopreservation review: Outcomes of medical oocyte cryopreservation and planned oocyte cryopreservation. Reprod. Biol. Endocrinol. 2022, 20, 10. [Google Scholar] [CrossRef]
- Human Fertilisation & Embryology Authority. Press Release: Age Is the Key Factor for Egg Freezing Success Says New Hfea Report, As Overall Treatment Numbers Remain Low; Human Fertilisation & Embryology Authority: London, UK, 2018.
- Doyle, J.O.; Richter, K.S.; Lim, J.; Stillman, R.J.; Graham, J.R.; Tucker, M.J. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil. Steril. 2016, 105, 459–466.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammarberg, K.; Kirkman, M.; Pritchard, N.; Hickey, M.; Peate, M.; McBain, J.; Agresta, F.; Bayly, C.; Fisher, J. Reproductive experiences of women who cryopreserved oocytes for non-medical reasons. Hum. Reprod. 2017, 32, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Human Fertilisation & Embryology Authority. Trends and Figures: 2010–2016. In Egg Freezing in Fertility Treatment; Human Fertilisation & Embryology Authority: London, UK, 2018. [Google Scholar]
- Johnston, M.; Fuscaldo, G.; Gwini, S.M.; Catt, S.; Richings, N.M. Financing future fertility: Women’s views on funding egg freezing. Reprod. Biomed. Soc. Online 2022, 14, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ben-Rafael, Z. The dilemma of social oocyte freezing: Usage rate is too low to make it cost effective. Reprod. Biomed. Online 2018, 37, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devine, K.; Mumford, S.L.; Goldman, K.N.; Hodes-Wertz, B.; Druckenmiller, S.; Propst, A.M.; Noyes, N. Baby budgeting: Oocyte cryopreservation in women delaying reproduction can reduce cost per live birth. Fertil. Steril. 2015, 103, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Goldman, R.H.; Racowsky, C.; Farland, L.V.; Munné, S.; Ribustello, L.; Fox, J.H. Predicting the likelihood of live birth for elective oocyte cryopreservation: A counseling tool for physicians and patients. Hum. Reprod. 2017, 32, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Varlas, V.N.; Bors, R.G.; Albu, D.; Penes, O.N.; Nasui, B.A.; Mehedintu, C.; Pop, A.L. Social Freezing: Pressing Pause on Fertility. Int. J. Environ. Res. Public Health 2021, 18, 8088. [Google Scholar] [CrossRef]
- Human Fertilisation & Embryology Authority. Trends and figures: 2019. In Fertility Treatment; Human Fertilisation & Embryology Authority: London, UK, 2021. [Google Scholar]
- Ho, J.R.; Woo, I.; Louie, K.; Salem, W.; Jabara, I.S.; Bendikson, A.K.; Paulson, R.J.; Chung, K. A comparison of live birth rates and perinatal outcomes between cryopreserved oocytes and cryopreserved embryos. J. Assist. Reprod. Genet. 2017, 34, 1359–1366. [Google Scholar] [CrossRef]



| Variables | Age, Less than 38 Years | Age, 38 Years or Above | p-Value |
|---|---|---|---|
| Number of patients returned back (n = 27) | 15 (56%) | 12 (44%) | NA |
| Mean eggs frozen | 12 | 9 | 0.41 |
| Average duration of storage (n = 27) | 5.46 years | 3.1 years | 0.70 |
| Survival rate | 119/176 (68%) | 138/170 (81%) | 0.73 |
| Fertilisation rate per oocyte injected | 116/155 (75%) | 60/102 (59%) | 0.26 |
| Pregnancy rate per ET | 9/16 (56%) | 2/7 (29%) | 0.22 |
| Live birth rate per ET | 6/16 (38%) | 2/7 (29%) | 0.77 |
| Articles | Mean Age | Women Returned | Oocyte Survival | Fertilisation Rate per Oocyte Injected | Fertilisation Rate per Oocyte Injected | Live Birth Rate per ET/per Women |
|---|---|---|---|---|---|---|
| Kasaven et al., 2022 [14] | 38 | 36/373 | - | 60% | - | 30% per ET |
| Harjee et al., 2022 [21] | 36.5 | 50/556 | - | 60% | - | 65% per ET |
| Avi Tsafrir et al., 2022 [15] | 37.9 | 57/446 | - | - | - | 27% per woman |
| Lh-Jane Yang et al., 2022 [16] | 38.1 | 68/921 | - | 74% | - | 39% per ET |
| Blakemore et al., 2021 [22] | 38.2 | 88/231 | 932/1256 | 70% | 642/932 | 27% per ET |
| Gurtin et al., 2019 [23] | 37.7 | 46 | - | - | - | 17% per woman |
| Wafi et al., 2019 [24] | 36 | 28/138 | - | - | - | 21% per woman |
| Cobo et al., 2018 [25] | 37.2 | 641/5289 | 4891/5830 | - | - | 39% per ET |
| Wennberg et al., 2018 [26] | 37 | 38/254 | 307/393 | 62% | 190/307 | 37% per ET |
| Nagy et al., 2017 [27] | 36.9 | 50 | 332/425 | - | - | 20% per ET |
| Average | 37.3 | 1006/8208 (12%) | 6462/7904 (82%) | 67% | 832/1239 (67%) |
| Outcomes | Current Study | Worldwide Data | Collated Data |
|---|---|---|---|
| Mean age at freezing | 37.1 years | 37.3 years | 37.2 years |
| Usage rate | 16% | 1006/8208 (12%) | 1031/8375 (12%) |
| Thaw/survival rate per oocyte frozen | 74% | 6462/7904 (82%) | 6508/8230 (79%) |
| Fertilisation rate per injected oocyte | 67% | 832/1239 (67%) | 977/1439 (68%) |
| Live birth rate per ET | 35% | 196/558 (35%) | 203/580 (35%) |
| Less than 38 years | 38% | 24/50 (48%) | 29/65 (45%) |
| 38 years of age and above | 29% | 20/94 (21%) | 22/101 (22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakkar, P.; Geary, J.; Stockburger, T.; Kaffel, A.; Kopeika, J.; El-Toukhy, T. Outcomes of Social Egg Freezing: A Cohort Study and a Comprehensive Literature Review. J. Clin. Med. 2023, 12, 4182. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12134182
Kakkar P, Geary J, Stockburger T, Kaffel A, Kopeika J, El-Toukhy T. Outcomes of Social Egg Freezing: A Cohort Study and a Comprehensive Literature Review. Journal of Clinical Medicine. 2023; 12(13):4182. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12134182
Chicago/Turabian StyleKakkar, Pragati, Joanna Geary, Tania Stockburger, Aida Kaffel, Julia Kopeika, and Tarek El-Toukhy. 2023. "Outcomes of Social Egg Freezing: A Cohort Study and a Comprehensive Literature Review" Journal of Clinical Medicine 12, no. 13: 4182. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12134182