Serological Responses and Predictive Factors of Booster COVID-19 Vaccines in Patients with Hematologic Malignancies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Laboratory Analyses and Clinical Parameters
2.3. Statistical Analysis
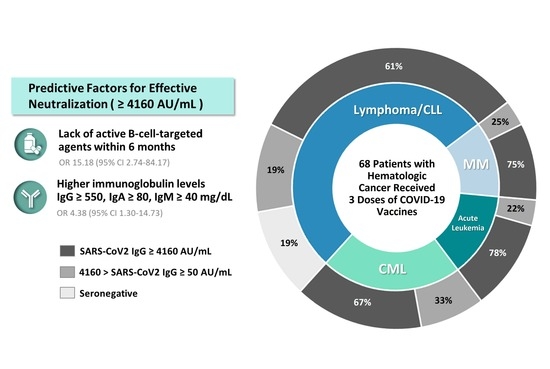
3. Results
3.1. Patient Characteristics
3.2. Serological Responses
3.3. Predictive Factors Associated with Effective Antibody Neutralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taiwan National Infectious Disease Statistics System. Available online: https://nidss.cdc.gov.tw/en/nndss/disease?id=19CoV (accessed on 20 March 2023).
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Pagano, L.; Salmanton-Garcia, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 168. [Google Scholar] [CrossRef]
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martin-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892. [Google Scholar] [CrossRef]
- Pagano, L.; Salmanton-Garcia, J.; Marchesi, F.; Blennow, O.; Gomes da Silva, M.; Glenthoj, A.; van Doesum, J.; Bilgin, Y.M.; Lopez-Garcia, A.; Itri, F.; et al. Breakthrough COVID-19 in vaccinated patients with hematologic malignancies: Results from the EPICOVIDEHA survey. Blood 2022, 140, 2773–2787. [Google Scholar] [CrossRef]
- Benda, M.; Mutschlechner, B.; Ulmer, H.; Grabher, C.; Severgnini, L.; Volgger, A.; Reimann, P.; Lang, T.; Atzl, M.; Huynh, M.; et al. Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients. Br. J. Haematol. 2021, 195, 523–531. [Google Scholar] [CrossRef]
- Herzog Tzarfati, K.; Gutwein, O.; Apel, A.; Rahimi-Levene, N.; Sadovnik, M.; Harel, L.; Benveniste-Levkovitz, P.; Bar Chaim, A.; Koren-Michowitz, M. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am. J. Hematol. 2021, 96, 1195–1203. [Google Scholar] [CrossRef]
- Uaprasert, N.; Pitakkitnukun, P.; Tangcheewinsirikul, N.; Chiasakul, T.; Rojnuckarin, P. Immunogenicity and risks associated with impaired immune responses following SARS-CoV-2 vaccination and booster in hematologic malignancy patients: An updated meta-analysis. Blood Cancer J. 2022, 12, 173. [Google Scholar] [CrossRef]
- Abid, M.B.; Rubin, M.; Ledeboer, N.; Szabo, A.; Longo, W.; Mohan, M.; Shah, N.N.; Fenske, T.S.; Abedin, S.; Runaas, L.; et al. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell 2022, 40, 340–342. [Google Scholar] [CrossRef]
- Bagacean, C.; Letestu, R.; Al-Nawakil, C.; Brichler, S.; Levy, V.; Sritharan, N.; Delmer, A.; Dartigeas, C.; Leblond, V.; Roos-Weil, D.; et al. Humoral response to mRNA anti-COVID-19 vaccines BNT162b2 and mRNA-1273 in patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 207–211. [Google Scholar] [CrossRef]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Osterlev, S.; Kragh, A.; Frederiksen, H.; Ditzel, H.J. Antibody responses following third mRNA COVID-19 vaccination in patients with cancer and potential timing of a fourth vaccination. Cancer Cell 2022, 40, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Rahav, G.; Levi, S.; Braester, A.; Itchaki, G.; Bairey, O.; Dally, N.; Shvidel, L.; Ziv-Baran, T.; Polliack, A.; et al. Efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL who failed standard 2-dose vaccination. Blood 2022, 139, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.; Redjoul, R.; Klemencie, M.; Labussiere Wallet, H.; Le Bourgeois, A.; D’Aveni, M.; Huynh, A.; Berceanu, A.; Marchand, T.; Chantepie, S.; et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood 2022, 139, 134–137. [Google Scholar] [CrossRef]
- Saiag, E.; Grupper, A.; Avivi, I.; Elkayam, O.; Ram, R.; Herishanu, Y.; Cohen, Y.; Perry, C.; Furer, V.; Katchman, H.; et al. The effect of a third-dose BNT162b2 vaccine on anti-SARS-CoV-2 antibody levels in immunosuppressed patients. Clin. Microbiol. Infect. 2022, 28, 735.e5–735.e8. [Google Scholar] [CrossRef] [PubMed]
- Susol, O.; Hajkova, B.; Zelena, H.; Hajek, R. Third dose of COVID-19 vaccine restores immune response in patients with haematological malignancies after loss of protective antibody titres. Br. J. Haematol. 2022, 197, 302–305. [Google Scholar] [CrossRef]
- Thompson, M.A.; Hallmeyer, S.; Fitzpatrick, V.E.; Liao, Y.; Mullane, M.P.; Medlin, S.C.; Copeland, K.; Weese, J.L. Real-World Third COVID-19 Vaccine Dosing and Antibody Response in Patients With Hematologic Malignancies. J. Patient Cent. Res. Rev. 2022, 9, 149–157. [Google Scholar] [CrossRef]
- Ollila, T.A.; Masel, R.H.; Reagan, J.L.; Lu, S.; Rogers, R.D.; Paiva, K.J.; Taher, R.; Burguera-Couce, E.; Zayac, A.S.; Yakirevich, I.; et al. Seroconversion and outcomes after initial and booster COVID-19 vaccination in adults with hematologic malignancies. Cancer 2022, 128, 3319–3329. [Google Scholar] [CrossRef]
- Haggenburg, S.; Hofsink, Q.; Lissenberg-Witte, B.I.; Broers, A.E.C.; van Doesum, J.A.; van Binnendijk, R.S.; den Hartog, G.; Bhoekhan, M.S.; Haverkate, N.J.E.; Burger, J.A.; et al. Antibody Response in Immunocompromised Patients With Hematologic Cancers Who Received a 3-Dose mRNA-1273 Vaccination Schedule for COVID-19. JAMA Oncol. 2022, 8, 1477–1483. [Google Scholar] [CrossRef]
- Huang, C.-F.; Jang, T.-Y.; Wu, P.-H.; Kuo, M.-C.; Yeh, M.-L.; Wang, C.-W.; Liang, P.-C.; Wei, Y.-J.; Hsu, P.-Y.; Huang, C.-I. Impact of comorbidities on the serological response to COVID-19 vaccination in a Taiwanese cohort. Virol. J. 2023, 20, 112. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Shorer Arbel, Y.; Scarfo, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Ikeda, D.; Terao, T.; Fukumoto, A.; Uesugi, Y.; Tabata, R.; Kuzume, A.; Tsushima, T.; Miura, D.; Narita, K.; Takeuchi, M.; et al. Antibody status following booster vaccination against SARS-CoV-2 virus in patients with haematologic malignancies. Br. J. Haematol. 2023, 200, 568–572. [Google Scholar] [CrossRef]
- Cook, L.B.; O’Dell, G.; Vourvou, E.; Palanicawandar, R.; Marks, S.; Milojkovic, D.; Apperley, J.F.; Loaiza, S.; Claudiani, S.; Bua, M.; et al. Third primary SARS-CoV-2 mRNA vaccines enhance antibody responses in most patients with haematological malignancies. Nat. Commun. 2022, 13, 6922. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Cesaro, S.; de Lavallade, H.; Di Blasi, R.; Einarsdottir, S.; Gallo, G.; Rieger, C.; Engelhard, D.; Lehrnbecher, T.; Ljungman, P.; et al. Vaccination of patients with haematological malignancies who did not have transplantations: Guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e188–e199. [Google Scholar] [CrossRef]
- Gurion, R.; Rozovski, U.; Itchaki, G.; Gafter-Gvili, A.; Leibovitch, C.; Raanani, P.; Ben-Zvi, H.; Szwarcwort, M.; Taylor-Abigadol, M.; Dann, E.J.; et al. Humoral serological response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica 2022, 107, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.; Delord, M.; Chbat, M.; Guemriche, A.; Merabet, F.; Roupie, A.L.; Lombion, N.; Farhat, H.; Longval, T.; Cabannes-Hamy, A.; et al. A third anti-SARS-CoV-2 mRNA dose does not overcome the pejorative impact of anti-CD20 therapy and/or low immunoglobulin levels in patients with lymphoma or chronic lymphocytic leukemia. Haematologica 2022, 107, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Maneikis, K.; Sablauskas, K.; Ringeleviciute, U.; Vaitekenaite, V.; Cekauskiene, R.; Kryzauskaite, L.; Naumovas, D.; Banys, V.; Peceliunas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Takata, T.; Suzumiya, J.; Ishikawa, T.; Takamatsu, Y.; Ikematsu, H.; Tamura, K. Attenuated antibody reaction for the primary antigen but not for the recall antigen of influenza vaccination in patients with non-Hodgkin B-cell lymphoma after the administration of rituximab-CHOP. J. Clin. Exp. Hematop. 2009, 49, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, M.; Boutin, M.; Laumaea, A.; Salaciak, M.; Mendoza, A.; Cassis, C.; Ajjamada, L.; Assouline, S.; Patenaude, F.; Clark, M.W.; et al. B-cell cytopenia and time to last B-cell-depleting therapy predict response to SARS-CoV-2 vaccines in patients with lymphoproliferative disorders. Vaccine 2022, 40, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Gaugler, B.; Gozlan, J.; Bouquet, L.; Fofana, D.; Siblany, L.; Eshagh, D.; Adotevi, O.; Laheurte, C.; Ricard, L.; et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021, 11, 142. [Google Scholar] [CrossRef]
- Haggenburg, S.; Lissenberg-Witte, B.I.; van Binnendijk, R.S.; den Hartog, G.; Bhoekhan, M.S.; Haverkate, N.J.E.; de Rooij, D.M.; van Meerloo, J.; Cloos, J.; Kootstra, N.A.; et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022, 6, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021, 34, 108728. [Google Scholar] [CrossRef] [PubMed]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8(+) T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Bilich, T.; Roerden, M.; Maringer, Y.; Nelde, A.; Heitmann, J.S.; Dubbelaar, M.L.; Peter, A.; Horber, S.; Bauer, J.; Rieth, J.; et al. Preexisting and Post-COVID-19 Immune Responses to SARS-CoV-2 in Patients with Cancer. Cancer Discov. 2021, 11, 1982–1995. [Google Scholar] [CrossRef]
- Jimenez, M.; Roldan, E.; Fernandez-Naval, C.; Villacampa, G.; Martinez-Gallo, M.; Medina-Gil, D.; Peralta-Garzon, S.; Pujadas, G.; Hernandez, C.; Pages, C.; et al. Cellular and humoral immunogenicity of the mRNA-1273 SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Adv. 2022, 6, 774–784. [Google Scholar] [CrossRef]
- Re, D.; Seitz-Polski, B.; Brglez, V.; Carles, M.; Graca, D.; Benzaken, S.; Liguori, S.; Zahreddine, K.; Delforge, M.; Bailly-Maitre, B.; et al. Humoral and cellular responses after a third dose of SARS-CoV-2 BNT162b2 vaccine in patients with lymphoid malignancies. Nat. Commun. 2022, 13, 864. [Google Scholar] [CrossRef]
- Corradini, P.; Agrati, C.; Apolone, G.; Mantovani, A.; Giannarelli, D.; Marasco, V.; Bordoni, V.; Sacchi, A.; Matusali, G.; Salvarani, C.; et al. Humoral and T-Cell Immune Response After 3 Doses of Messenger RNA Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines in Fragile Patients: The Italian VAX4FRAIL Study. Clin. Infect. Dis. 2023, 76, e426–e438. [Google Scholar] [CrossRef]
- Langerbeins, P.; Hallek, M. COVID-19 in patients with hematologic malignancy. Blood 2022, 140, 236–252. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/prevention-of-sars-cov-2/ (accessed on 6 March 2023).
- Patel, P.; Twentyman, E.; Koumans, E.; Rosenblum, H.; Griffin-Blake, S.; Jackson, B.; Vagi, S. Information for Persons Who Are Immunocompromised Regarding Prevention and Treatment of SARS-CoV-2 Infection in the Context of Currently Circulating Omicron Sublineages—United States, January 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 128–131. [Google Scholar] [CrossRef] [PubMed]
- FDA Announces Evusheld Is Not Currently Authorized for Emergency Use in the U.S. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us (accessed on 26 January 2023).




| Lymphoma and CLL (n = 36) | Chronic Myeloid Leukemia (n = 15) | Acute Leukemia (n = 9) | Multiple Myeloma (n = 8) | p-Value | |
|---|---|---|---|---|---|
| Age, median (range), years | 62 (26–78) | 56 (27–71) | 56 (27–71) | 73.5 (52–75) | 0.012 * |
| Male gender, n (%) | 18 (50.0%) | 5 (33.3%) | 4 (44.4%) | 5 (62.5%) | 0.562 |
| BMI, median (range), kg/m2 | 24.2 (18.1–35.0) | 23.7 (19.3–33.2) | 24.7 (20.5–30.9) | 23.6 (16.5–30.0) | 0.667 |
| Comorbidities, n (%) | 27 (75.0%) | 5 (33.3%) | 5 (55.6%) | 5 (62.5%) | 0.047 * |
| Hypertension | 14 (38.9%) | 2 (13.3%) | 0 (0.0%) | 3 (37.5%) | |
| Diabetes | 7 (19.4%) | 1 (6.7%) | 3 (33.3%) | 2 (25.0%) | |
| Chronic kidney disease | 4 (11.1%) | 2 (13.3%) | 0 (0.0%) | 4 (50.0%) | |
| Chronic liver disease 1 | 11 (30.6%) | 1 (6.7%) | 2 (22.2%) | 0 (0.0%) | |
| Cardiovascular disease 2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (12.5%) | |
| Cerebrovascular disease | 1 (2.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Peptic ulcer disease | 5 (13.9%) | 1 (6.7%) | 0 (0.0%) | 1 (12.5%) | |
| Charlson comorbidity index, median (range) | 4 (2–10) | 3 (2–5) | 3 (2–5) | 3 (1–7) | 0.206 |
| Active cancer, n (%) | 21 (58.3%) | 15 (100.0%) | 2 (22.2%) | 8 (100.0%) | <0.001 *** |
| Active treatment within 6 months, n (%) | 14 (38.9%) | 15 (100.0%) | 3 (33.3%) | 8 (100.0%) | <0.001 *** |
| IV chemotherapy | 12 (33.3%) | 0 (0.0%) | 2 (22.2%) | 0 (0.0%) | |
| B-cell-targeted agent 3 | 12 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Immunomodulatory drug | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 8 (100.0%) | |
| BCR-ABL TKI | 0 (0.0%) | 15 (100.0%) | 1 (11.1%) | 0 (0.0%) | |
| Anti-CD38 monoclonal antibodies | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (25.0%) | |
| Glucocorticoids | 12 (33.3%) | 1 (6.7%) | 1 (11.1%) | 8 (100.0%) | |
| HSCT, n (%) | 2 (5.6%) | 0 (0.0%) | 2 (22.2%) | 3 (37.5%) | 0.015 * |
| First and Second vaccinations, n (%) | 0.913 | ||||
| AZ-based | 9 (25.0%) | 4 (26.7%) | 2 (22.2%) | 2 (25.0%) | |
| mRNA-based | 24 (66.7%) | 10 (66.7%) | 7 (77.8%) | 5 (62.5%) | |
| MVC-based | 1 (2.8%) | 1 (6.7%) | 0 (0.0%) | 1 (12.5%) | |
| Mixed 4 | 2 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Booster vaccination, n (%) | 0.503 | ||||
| mRNA | 33 (91.7%) | 12 (80.0%) | 8 (88.9%) | 6 (75.0%) | |
| Medigen | 3 (5.6%) | 3 (20.0%) | 1 (11.1%) | 2 (25.0%) |
| Variable | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age < 65 years | 2.030 (0.722–5.704) | 0.179 | ||
| Female gender | 1.779 (0.645–4.907) | 0.266 | ||
| Without hypertension | 1.648 (0.552–4.926) | 0.371 | ||
| Without diabetes | 0.842 (0.229–3.097) | 0.796 | ||
| Without chronic kidney disease | 3.618 (0.905–14.463) | 0.069 | 1.939 (0.353–10.645) | 0.446 |
| Without chronic liver disease | 0.737 (0.203–2.669) | 0.642 | ||
| Charlson comorbidity index < 4 | 1.488 (0.536–4.131) | 0.446 | ||
| mRNA-based first and second vaccines | 1.582 (0.550–4.553) | 0.395 | ||
| Inactive cancer | 3.167 (0.924–10.857) | 0.067 | 0.985 (0.229–4.235) | 0.984 |
| Lack of active B-cell-targeted agent | 16.538 (3.208–85.261) | 0.001 ** | 15.177 (2.737–84.168) | 0.002 ** |
| Lack of active IV chemotherapy | 7.885 (2.113–29.418) | 0.002 ** | 2.053 (0.327–12.876) | 0.443 |
| Lack of active glucocorticoids | 2.833 (0.978–8.209) | 0.055 | 0.502 (0.099–2.550) | 0.406 |
| BCR-ABL TKI | 0.917 (0.290–2.902) | 0.882 | ||
| Immunomodulatory drug | 1.615 (0.299–8.719) | 0.577 | ||
| IgG ≥550, IgA ≥80, IgM ≥40 mg/dL | 4.808 (1.635–14.139) | 0.004 ** | 4.375 (1.299–14.731) | 0.017 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-T.; Lee, C.-P.; Chen, T.-Y.; Liu, Y.-C.; Cho, S.-F.; Du, J.-S.; Yu, M.-L.; Huang, C.-F.; Wang, S.-F.; Hsiao, H.-H. Serological Responses and Predictive Factors of Booster COVID-19 Vaccines in Patients with Hematologic Malignancies. J. Clin. Med. 2023, 12, 5647. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12175647
Huang C-T, Lee C-P, Chen T-Y, Liu Y-C, Cho S-F, Du J-S, Yu M-L, Huang C-F, Wang S-F, Hsiao H-H. Serological Responses and Predictive Factors of Booster COVID-19 Vaccines in Patients with Hematologic Malignancies. Journal of Clinical Medicine. 2023; 12(17):5647. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12175647
Chicago/Turabian StyleHuang, Chien-Tzu, Ching-Ping Lee, Tzu-Yin Chen, Yi-Chang Liu, Shih-Feng Cho, Jeng-Shiun Du, Ming-Lung Yu, Chung-Feng Huang, Sheng-Fan Wang, and Hui-Hua Hsiao. 2023. "Serological Responses and Predictive Factors of Booster COVID-19 Vaccines in Patients with Hematologic Malignancies" Journal of Clinical Medicine 12, no. 17: 5647. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12175647