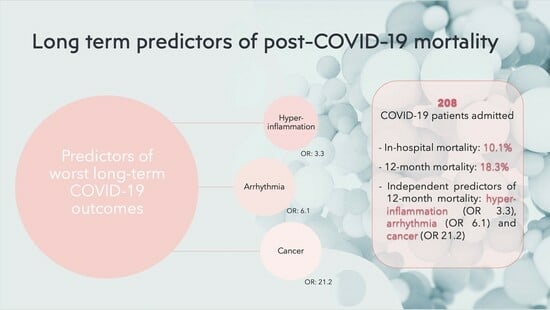
Long-Term Prognosis among COVID-19 Patients: The Predictive Role Played by Hyperinflammation and Arrhythmic Disorders in Fatal Outcome
Abstract
:1. Background
2. Materials and Methods
Statistical Analysis
3. Results
3.1. At Discharge (Baseline)
3.1.1. Cardiac Telemetric Monitoring
3.1.2. Laboratory Assays
3.1.3. Lung and Cardiac Instrumental Investigations
3.2. At One-Year Follow-Up
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Weekly Epidemiological Update on COVID-19—Weekly Epidemiological Update on COVID-19—25 May 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19%2D-25-may-2023 (accessed on 30 May 2023).
- Ortega-Paz, L.; Arévalos, V.; Fernández-Rodríguez, D.; Jiménez-Díaz, V.; Bañeras, J.; Campo, G.; Rodríguez-Santamarta, M.; Díaz, J.F.; Scardino, C.; Gómez-Álvarez, Z.; et al. CV COVID-19 registry investigators. One-year cardiovascular outcomes after coronavirus disease 2019: The cardiovascular COVID-19 registry. PLoS ONE 2022, 17, e0279333. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818, Erratum in JAMA Cardiol. 2020, 5, 848. [Google Scholar] [CrossRef]
- Ingul, C.B.; Grimsmo, J.; Mecinaj, A.; Trebinjac, D.; Berger Nossen, M.; Andrup, S.; Grenne, B.; Dalen, H.; Einvik, G.; Stavem, K.; et al. Cardiac Dysfunction and Arrhythmias 3 Months After Hospitalization for COVID-19. J. Am. Heart Assoc. 2022, 11, e023473. [Google Scholar] [CrossRef]
- La Via, L.; Dezio, V.; Santonocito, C.; Astuto, M.; Morelli, A.; Huang, S.; Vieillard-Baron, A.; Sanfilippo, F. Full and simplified assessment of left ventricular diastolic function in covid-19 patients admitted to ICU: Feasibility, incidence, and association with mortality. Echocardiography 2022, 39, 1391–1400. [Google Scholar] [CrossRef]
- Huang, S.; Vieillard-Baron, A.; Evrard, B.; Prat, G.; Chew, M.S.; Balik, M.; Clau-Terré, F.; De Backer, D.; Mekontso Dessap, A.; Orde, S.; et al. Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: Post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023, 49, 946–956. [Google Scholar] [CrossRef]
- Cozzolino, D.; Romano, C.; Nevola, R.; Marrone, A.; Umano, G.R.; Cuomo, G.; Rinaldi, L.; Adinolfi, L.E.; Vanvitelli COVID Collaborators. COVID-19 and arrhythmia: The factors associated and the role of myocardial electrical impulse propagation. An observational study based on cardiac telemetric monitoring. Front. Cardiovasc. Med. 2022, 9, 912474. [Google Scholar] [CrossRef]
- Cho, J.H.; Namazi, A.; Shelton, R.; Ramireddy, A.; Ehdaie, A.; Shehata, M.; Wang, X.; Marbán, E.; Chugh, S.S.; Cingolani, E. Cardiac arrhythmias in hospitalized patients with COVID-19: A prospective observational study in the western United States. PLoS ONE 2020, 15, e0244533. [Google Scholar] [CrossRef]
- Peltzer, B.; Manocha, K.K.; Ying, X.; Kirzner, J.; Ip, J.E.; Thomas, G.; Liu, C.F.; Markowitz, S.M.; Lerman, B.B.; Safford, M.M.; et al. Arrhythmic Complications of Patients Hospitalized With COVID-19: Incidence, Risk Factors, and Outcomes. Circ. Arrhythm. Electrophysiol. 2020, 13, e009121. [Google Scholar] [CrossRef]
- Romano, C.; Cozzolino, D.; Cuomo, G.; Abitabile, M.; Carusone, C.; Cinone, F.; Nappo, F.; Nevola, R.; Sellitto, A.; Auricchio, A.; et al. Prediction of SARS-CoV-2-Related Lung Inflammation Spreading by V:ERITAS (Vanvitelli Early Recognition of Inflamed Thoracic Areas Spreading). J. Clin. Med. 2022, 11, 2434. [Google Scholar] [CrossRef]
- Nevola, R.; Marrone, A.; Cozzolino, D.; Cuomo, G.; Romano, C.P.; Rinaldi, L.; Aprea, C.; Padula, A.; Ranieri, R.; Gjeloshi, K.; et al. Predictors of in-hospital mortality of COVID-19 patients and the role of telemetry in an internal medicine ward during the third phase of the pandemic. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Aleksova, A.; Fluca, A.L.; Gagno, G.; Pierri, A.; Padoan, L.; Derin, A.; Moretti, R.; Noveska, E.A.; Azzalini, E.; D’Errico, S.; et al. Long-term effect of SARS-CoV-2 infection on cardiovascular outcomes and all-cause mortality. Life Sci. 2022, 310, 121018. [Google Scholar] [CrossRef]
- Ronco, C.; Bagshaw, S.M.; Bellomo, R.; Clark, W.R.; Husain-Syed, F.; Kellum, J.A.; Ricci, Z.; Rimmelé, T.; Reis, T.; Ostermann, M. Extracorporeal Blood Purification and Organ Support in the Critically Ill Patient during COVID-19 Pandemic: Expert Review and Recommendation. Blood Purif. 2021, 50, 17–27. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Martucci, G.; La Via, L.; Cuttone, G.; Dimarco, G.; Pulizzi, C.; Arcadipane, A.; Astuto, M. Hemoperfusion and blood purification strategies in patients with COVID-19: A systematic review. Artif. Organs 2021, 45, 1466–1476. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Bogossian, H.; Linz, D.; Heijman, J.; Bimpong-Buta, N.Y.; Bandorski, D.; Frommeyer, G.; Erkapic, D.; Seyfarth, M.; Zarse, M.; Crijns, H.J. QTc evaluation in patients with bundle branch block. Int. J. Cardiol. Heart Vasc. 2020, 30, 100636. [Google Scholar] [CrossRef]
- Indik, J.H.; Pearson, E.C.; Fried, K.; Woosley, R.L. Bazett and Fridericia QT correction formulas interfere with measurement of drug-induced changes in QT interval. Heart Rhythm 2006, 3, 1003–1007. [Google Scholar] [CrossRef]
- Balacchi, C.; Brandi, N.; Ciccarese, F.; Coppola, F.; Lucidi, V.; Bartalena, L.; Parmeggiani, A.; Paccapelo, A.; Golfieri, R. Comparing the first and the second waves of COVID-19 in Italy: Differences in epidemiological features and CT findings using a semi-quantitative score. Emerg. Radiol. 2021, 28, 1055–1061. [Google Scholar] [CrossRef]
- Lugara, M.; Oliva, G.; Pafundi, P.C.; Tamburrini, S.; Nevola, R.; Gjeloshi, K.; Ricozzi, C.; Imbriani, S.; Padula, A.; Aprea, C.; et al. Clinical application of lung ultrasound score on COVID-19 setting: A regional experience in Southern Italy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3623–3631. [Google Scholar] [CrossRef]
- Douglas, P.S.; Carabello, B.A.; Lang, R.M.; Lopez, L.; Pellikka, P.A.; Picard, M.H.; Thomas, J.D.; Varghese, P.; Wang, T.Y.; Weissman, N.J.; et al. 2019 ACC/AHA/ASE Key Data Elements and Definitions for Transthoracic Echocardiography: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) and the American Society of Echocardiography. Circ. Cardiovasc. Imaging 2019, 12, e000027. [Google Scholar] [CrossRef]
- Nevola, R.; Russo, A.; Scuotto, S.; Imbriani, S.; Aprea, C.; Abitabile, M.; Beccia, D.; Brin, C.; Carusone, C.; Cinone, F.; et al. Non-invasive respiratory support in SARS-CoV-2 related acute respiratory distress syndrome: When is it most appropriate to start treatment? Respir. Res. 2022, 23, 327. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Wang, X.Q.; Iwashyna, T.J.; Prescott, H.C. Readmission and Death After Initial Hospital Discharge Among Patients with COVID-19 in a Large Multihospital System. JAMA 2021, 325, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Takagi, H.; Ishikawa, K.; Kuno, T. Myocardial injury characterized by elevated cardiac troponin and in-hospital mortality of COVID-19: An insight from a meta-analysis. J. Med. Virol. 2021, 93, 51–55. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Kunichoff, D.; Garshick, M.; Shah, B.; Pillinger, M.; Hochman, J.S.; Berger, J.S. C-reactive protein and clinical outcomes in patients with COVID-19. Eur. Heart J. 2021, 42, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Siddiqi, H.; Zhou, G.; Vieira, J.; Kim, A.; Rutherford, H.; Mitre, X.; Feeley, M.; Oganezova, K.; Varshney, A.S.; et al. Relationship Between Myocardial Injury During Index Hospitalization for SARS-CoV-2 Infection and Longer-Term Outcomes. J. Am. Heart Assoc. 2022, 11, e022010. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; van der Wal, A.C.; Aubry, M.C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef]
- Renda, G.; Ricci, F.; Spinoni, E.G.; Grisafi, L.; D’Ardes, D.; Mennuni, M.; Tana, C.; Rognoni, A.; Bellan, M.; Sainaghi, P.P.; et al. Predictors of Mortality and Cardiovascular Outcome at 6 Months after Hospitalization for COVID-19. J. Clin. Med. 2022, 11, 729. [Google Scholar] [CrossRef]
- Dherange, P.; Lang, J.; Qian, P.; Oberfeld, B.; Sauer, W.H.; Koplan, B.; Tedrow, U. Arrhythmias and COVID-19: A Review. JACC Clin. Electrophysiol. 2020, 6, 1193–1204. [Google Scholar] [CrossRef]
- Cubeddu, L.X. QT prolongation and fatal arrhythmias: A review of clinical implications and effects of drugs. Am. J. Ther. 2003, 10, 452–457. [Google Scholar] [CrossRef]
- Nevola, R.; Feola, G.; Ruocco, R.; Russo, A.; Villani, A.; Fusco, R.; De Pascalis, S.; Del Core, M.; Cirigliano, G.; Pisaturo, M.; et al. Mortality and risk factors of vaccinated and unvaccinated frail patients with COVID-19 treated with anti-SARS-CoV-2 monoclonal antibodies: A real-world study. Int. J. Infect. Dis. 2023, 131, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Behers, B.J.; Patrick, G.A.; Jones, J.M.; Carr, R.A.; Behers, B.M.; Melchor, J.; Rahl, D.E.; Guerriero, T.D.; Zhang, H.; Ozkardes, C.; et al. Myocarditis Following COVID-19 Vaccination: A Systematic Review of Case Reports. Yale J. Biol. Med 2022, 95, 237–247. [Google Scholar] [PubMed]
- Bozkurt, B. Shedding Light on Mechanisms of Myocarditis With COVID-19 mRNA Vaccines. Circulation 2023, 147, 877–880. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n 187) | Reference Range/Values | |
|---|---|---|
| General features | ||
| Sex (M/F) | 113/74 | - |
| Age (years) | 73 (68–84) | - |
| Current tobacco user n (%) | 83 (44.4) | - |
| Obesity n (%) | 42 (22.5) | - |
| Diabetes mellitus n (%) | 42 (22.5) | - |
| Chronic liver disease n (%) | 20 (10.7) | - |
| Chronic kidney disease n (%) | 15 (8.0) | - |
| COPD n (%) | 26 (13.9) | - |
| Hyperlipidemia n (%) | 23 (12.3) | - |
| Auto-immune disease n (%) | 14 (7.5) | - |
| Active malignancy n (%) | 8 (4.3) | - |
| Prior organ transplantation n (%) | 2 (1.1) | - |
| Rare disease n (%) | 8 (4.3) | - |
| Arterial hypertension n (%) | 98 (52.4) | - |
| Heart failure n (%) | 8 (4.3) | - |
| Coronary artery disease n (%) | 24 (12.8) | - |
| Arrhythmia n (%) | 60 (32.1) | - |
| ICD/PPM n (%) | 7 (3.7) | - |
| Comorbidities | 4 (3–4) | - |
| Laboratory findings | ||
| Sodium (mmol/L) | 137 (135–140) | 135–146 |
| Potassium (mmol/L) | 4.2 (3.8–4.6) | 3.5–5.3 |
| Magnesium (mg/dL) | 2.0 (1.8–2.2) | 1.6–2.6 |
| Calcium (mg/dL) | 8.7 (8.4–9.0) | 8.6–10.2 |
| Creatinine (mg/dL) | 0.83 (0.74–1.12) | 0.51–0.95 |
| GFR (mL/min/1.73 m2) | 58.0 (49.0–82.0) | ≥60 |
| B-type natriuretic peptide (pg/mL) | 55.3 (8.0–217.5) | ≤125 |
| Troponin (ng/mL) | 5.1 (2.5–24.4) | ≤33 |
| C-reactive protein (mg/dL) | 4.15 (1.55–11.6) | ≤5 |
| Ferritin (ng/mL) | 1018 (201–2052) | 13–150 |
| Interleukin-6 (pg/mL) | 30 (16–107) | ≤5 |
| D-dimer (ng/mL) | 1200 (545–2280) | ≤500 * |
| Hemoglobin (g/dL) | 12.3 (9.9–12.3) | 13–15/12.5–14.5 (M/F) |
| Instrumental findings | ||
| Left ventricle ejection fraction (%) | 54 (50–55) | 55–65 |
| Lung computed tomography score | 8.5 (6–12) | 0 |
| Lung ultrasound score | 14 (7–23) | 0 |
| QT (ms) | 361 (345–363) | 340–430 |
| QTc (ms) | 421 (391–462) | 340–440/340–460 (M/F) |
| QTc dispersion (ms) | 72 (51–100) | ≤80 |
| Survivors (n 170) | Non-Survivors (n 17) | p Value | |
|---|---|---|---|
| General features | |||
| Sex (M, %) | 58.0 | 47.1 | 0.44 |
| Age (years) | 61 (52–71) | 73 (68–84) | 0.0001 |
| Current tobacco n (%) | 75 (44.1) | 8 (47.1) | 0.80 |
| Obesity n (%) | 39 (22.9) | 3 (17.6) | 0.77 |
| Diabetes mellitus n (%) | 37 (21.8) | 5 (29.4) | 0.54 |
| Active malignancy n (%) | 4 (2.3) | 4 (23.5) | 0.0004 |
| Arterial hypertension n (%) | 86 (50.6) | 12 (70.6) | 0.14 |
| Heart failure n (%) | 6 (3.6) | 3 (17.7) | 0.04 |
| Coronary artery disease n (%) | 20 (11.8) | 4 (23.5) | 0.24 |
| Arrhythmia n (%) | 48 (28.2) | 12 (70.6) | 0.0008 |
| Comorbidities | 2 (1–3) | 4 (3–4) | 0.003 |
| Laboratory findings | |||
| Creatinine (mg/dL) | 0.81 (0.72–0.93) | 0.83 (0.74–1.12) | 0.50 |
| GFR (mL/min/1.73 m2) | 92.0 (75.0–102.0) | 58.0 (49.0–82.0) | 0.0003 |
| B-type natriuretic peptide (pg/mL) | 18.0 (10.0–43.0) | 59.5 (47.0–281.0) | 0.001 |
| Troponin (ng/mL) | 3.0 (2.0–8.0) | 15.0 (6.0–89.5) | 0.0004 |
| C-reactive protein (mg/dL) | 0.66 (0.27–1.80) | 6.90 (3.20–11.70) | <0.0001 |
| Ferritin (ng/mL) | 479 (224–746) | 1018 (201–2052) | 0.10 |
| Interleukin-6 (pg/mL) | 20.9 (8.5–39.0) | 30.0 (16–107.0) | 0.29 |
| D-dimer (ng/mL) | 610 (346–1090) | 1200 (545–2280) | 0.01 |
| Hemoglobin (g/dL) | 13.4 (12.1–14.3) | 12.3 (9.9–13.8) | 0.056 |
| Instrumental findings | |||
| Left ventricle ejection fraction (%) | 55 (51–55) | 53 (50–55) | 0.29 |
| Lung computed tomography score | 9 (6–12) | 8.5 (6.0–12.0) | 0.87 |
| Lung ultrasound score | 12 (8–18) | 14 (7–23) | 0.50 |
| QT (ms) | 360 (320–405) | 363 (351–365) | 0.95 |
| QTc (ms) | 393 (380–415) | 421 (393–461) | 0.02 |
| QTc dispersion (ms) | 70 (50–83) | 72 (51–100) | 0.09 |
| Medical treatments | |||
| Proton-pump inhibitors n (%) | 80 (47.1) | 8 (47.1) | 1.00 |
| Antihypertensive drugs n (%) | 86 (50.6) | 12 (70.6) | 0.14 |
| Antihyperglycemic drugs n (%) | 36 (21.2) | 5 (29.4) | 0.65 |
| Statins n (%) | 20 (11.8) | 3 (17.6) | 0.47 |
| Class I, III anti-arrhythmic drugs n (%) | 45 (26.5) | 7 (41.2) | 0.19 |
| Antiplatelet drugs n (%) | 35 (20.6) | 5 (29.4) | 0.61 |
| Oral anticoagulant drugs n (%) | 21 (12.4) | 3 (17.6) | 0.52 |
| Loop diuretics n (%) | 19 (11.2) | 4 (23.5) | 0.14 |
| ARNI n (%) | 5 (2.9) | 2 (11.7) | 0.75 |
| Anti-inflammatory drugs n (%) | 19 (11.2) | 3 (17.6) | 0.49 |
| Inhaled bronchodilator drugs n (%) | 23 (13.5) | 3 (17.6) | 0.59 |
| Antibiotics n (%) | 19 (11.2) | 3 (17.6) | 0.49 |
| Respiratory clinical findings | |||
| ARDS n (%) | 117 (68.8) | 14 (82.3) | 0.24 |
| Need for CPAP/NIV | 58 (34.1) | 5 (29.4) | 0.69 |
| Need for OTI and IMV (ICU admission) | 0 (1) | 1 (5.9) | 0.09 |
| Univariate Analysis | |||
|---|---|---|---|
| OR | CI | p Value | |
| Age | 1.10 | 1.04–1.14 | 0.0003 |
| Active malignancy | 17.2 | 4.1–72.5 | 0.0001 |
| Heart failure | 5.8 | 1.3–25.8 | 0.02 |
| Arrhythmia | 6.05 | 2.01–18.09 | 0.0013 |
| Number of comorbidities | 2.28 | 0.91–5.67 | 0.08 |
| Glomerular filtration rate | 0.28 | 0.12–0.67 | 0.004 |
| B-type natriuretic peptide | 1.95 | 1.30–2.92 | 0.001 |
| Troponin | 2.33 | 1.51–3.62 | 0.0002 |
| C-reactive protein | 3.13 | 1.89–5.17 | <0.0001 |
| D-dimer | 2.11 | 1.17–3.80 | <0.0001 |
| QTc | 21.0 | <0.1–152 | 0.76 |
| Multivariate Analysis | |||
|---|---|---|---|
| OR | CI | p Value | |
| Age | 1.14 | 1.00–1.30 | 0.051 |
| Active malignancy | 21.2 | 4.1–111.7 | 0.0003 |
| Heart failure | 3.6 | 0.6–20.9 | 0.15 |
| Arrhythmia | 6.1 | 1.8–20.9 | 0.004 |
| Glomerular filtration rate | 1.7 | 0.2–15.2 | 0.62 |
| B-type natriuretic peptide | 1.6 | 0.7–3.5 | 0.29 |
| Troponin | 1.9 | 0.8–4.9 | 0.16 |
| C-reactive protein | 3.3 | 1.6–6.7 | 0.0009 |
| D-dimer | 0.9 | 0.4–3.4 | 0.88 |
| Within 3 Months from Hospital Discharge | |
| Patient #1 | Acute respiratory distress |
| Patient #2 | Cancer of colon |
| Patient #3 | Inflammatory bowel disease |
| Patient #4 | Bowel obstruction |
| Patient #5 | Prostate cancer |
| Patient #6 | Hemorrhagic stroke |
| Patient #7 | Severe sepsis |
| Patient #8 | Cholangiocarcinoma |
| Patient #9 | Decompensated heart failure |
| Patient #10 | Acute myocardial infarction |
| Between 4 and 6 months from hospital discharge | |
| Patient #11 | Ischemic stroke |
| Patient #12 | Cancer of lung |
| Patient #13 | Hemmorrhagic stroke |
| Patient #14 | Serious arrhythmia |
| Between 7 and 9 months from hospital discharge | |
| Patient #15 | Cancer of colon |
| Patient #16 | Serious arrhythmia |
| Patient #17 | Serious arrhythmia |
| Between 10 and 12 months from hospital discharge | |
| None | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzolino, D.; Romano, C.; Sardu, C.; Nevola, R.; Umano, G.R.; Rinaldi, L.; Adinolfi, L.E.; Catalini, C.; Marrone, A.; Municinò, M.; et al. Long-Term Prognosis among COVID-19 Patients: The Predictive Role Played by Hyperinflammation and Arrhythmic Disorders in Fatal Outcome. J. Clin. Med. 2023, 12, 5691. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12175691
Cozzolino D, Romano C, Sardu C, Nevola R, Umano GR, Rinaldi L, Adinolfi LE, Catalini C, Marrone A, Municinò M, et al. Long-Term Prognosis among COVID-19 Patients: The Predictive Role Played by Hyperinflammation and Arrhythmic Disorders in Fatal Outcome. Journal of Clinical Medicine. 2023; 12(17):5691. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12175691
Chicago/Turabian StyleCozzolino, Domenico, Ciro Romano, Celestino Sardu, Riccardo Nevola, Giuseppina Rosaria Umano, Luca Rinaldi, Luigi Elio Adinolfi, Christian Catalini, Aldo Marrone, Maurizio Municinò, and et al. 2023. "Long-Term Prognosis among COVID-19 Patients: The Predictive Role Played by Hyperinflammation and Arrhythmic Disorders in Fatal Outcome" Journal of Clinical Medicine 12, no. 17: 5691. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12175691