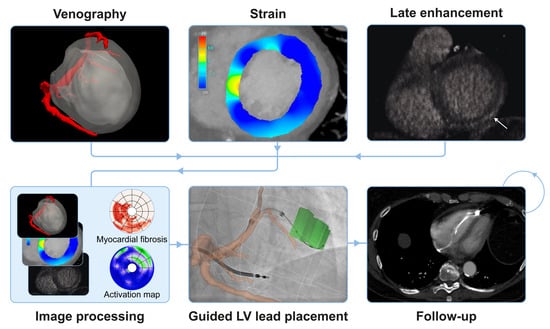
Cardiac CT in CRT as a Singular Imaging Modality for Diagnosis and Patient-Tailored Management
Abstract
:1. Introduction
2. Relevant Advances in Cardiac Computed Tomography
3. Multipurpose Cardiac Computed Tomography for Cardiac Resynchronization Therapy Patients
3.1. Identifying Patients with Cardiac Computed Tomography That May Benefit from Cardiac Resynchronization Therapy
3.1.1. Evaluation of the Coronary Artery System
3.1.2. Assessment of Ventricular Volume and Function
3.2. Comprehensive Assessment of Patients Eligible for Cardiac Resynchronization Therapy with Cardiac Computed Tomography
3.2.1. Coronary Venous System
3.2.2. Scar Identification
3.2.3. Extracellular Volume
3.2.4. Strain Measurement
3.2.5. Phrenic Nerve Identification
3.3. Cardiac Computed Tomography Guided Cardiac Resynchronization Therapy
3.3.1. Guided Left Ventricular Lead Placement
3.3.2. Cardiac Computed Tomography to Determine the Method of Left Ventricular Lead Delivery
3.3.3. Follow-up after Guided Cardiac Resynchronization Therapy
4. Implications
Financial and Safety Considerations
5. Limitations and Future Perspective
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vernooy, K.; van Deursen, C.J.M.; Strik, M.; Prinzen, F.W. Strategies to improve cardiac resynchronization therapy. Nat. Rev. Cardiol. 2014, 11, 481–493. [Google Scholar] [CrossRef]
- Maass, A.H.; Vernooy, K.; Wijers, S.C.; van’t Sant, J.; Cramer, M.J.; Meine, M.; Allaart, C.P.; De Lange, F.J.; Prinzen, F.W.; Gerritse, B.; et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: Results from the Markers and Response to CRT (MARC) study. EP Eur. 2018, 20, e1–e10. [Google Scholar] [CrossRef]
- Hu, X.; Xu, H.; Hassea, S.R.A.; Qian, Z.; Wang, Y.; Zhang, X.; Hou, X.; Zou, J. Comparative efficacy of image-guided techniques in cardiac resynchronization therapy: A meta-analysis. BMC Cardiovasc. Disord. 2021, 21, 255. [Google Scholar] [CrossRef]
- Behon, A.; Merkel, E.D.; Schwertner, W.R.; Kuthi, L.K.; Veres, B.; Masszi, R.; Kovács, A.; Lakatos, B.K.; Zima, E.; Gellér, L.; et al. Lateral left ventricular lead position is superior to posterior position in long-term outcome of patients who underwent cardiac resynchronization therapy. ESC Heart Fail. 2020, 7, 3374–3382. [Google Scholar] [CrossRef]
- Kutyifa, V.; Kosztin, A.; Klein, H.U.; Biton, Y.; Nagy, V.K.; Solomon, S.D.; McNitt, S.; Zareba, W.; Goldenberg, I.; Roka, A.; et al. Left Ventricular Lead Location and Long-Term Outcomes in Cardiac Resynchronization Therapy Patients. JACC Clin. Electrophysiol. 2018, 4, 1410–1420. [Google Scholar] [CrossRef]
- Salden, O.A.E.; van den Broek, H.T.; van Everdingen, W.M.; Hoesein, F.A.A.M.; Velthuis, B.K.; Doevendans, P.A.; Cramer, M.-J.; Tuinenburg, A.E.; Leufkens, P.; van Slochteren, F.J.; et al. Multimodality imaging for real-time image-guided left ventricular lead placement during cardiac resynchronization therapy implantations. Int. J. Cardiovasc. Imaging 2019, 35, 1327–1337. [Google Scholar] [CrossRef]
- Tada, T.; Osuda, K.; Nakata, T.; Muranaka, I.; Himeno, M.; Muratsubaki, S.; Murase, H.; Sato, K.; Hirose, M.; Fukuma, T. A novel approach to the selection of an appropriate pacing position for optimal cardiac resynchronization therapy using CT coronary venography and myocardial perfusion imaging: FIVE STaR method (fusion image using CT coronary venography and perfusion SPEC. J. Nucl. Cardiol. 2021, 28, 1438–1445. [Google Scholar] [CrossRef]
- Asferg, C.; Usinger, L.; Kristensen, T.S.; Abdulla, J. Accuracy of multi-slice computed tomography for measurement of left ventricular ejection fraction compared with cardiac magnetic resonance imaging and two-dimensional transthoracic echocardiography: A systematic review and meta-analysis. Eur. J. Radiol. 2012, 81, e757–e762. [Google Scholar] [CrossRef]
- Kaniewska, M.; Schuetz, G.M.; Willun, S.; Schlattmann, P.; Dewey, M. Noninvasive evaluation of global and regional left ventricular function using computed tomography and magnetic resonance imaging: A meta-analysis. Eur. Radiol. 2017, 27, 1640–1659. [Google Scholar] [CrossRef]
- Sharma, A.; Einstein, A.J.; Vallakati, A.; Arbab-Zadeh, A.; Mukherjee, D.; Lichstein, E. Meta-analysis of global left ventricular function comparing multidetector computed tomography with cardiac magnetic resonance imaging. Am. J. Cardiol. 2014, 113, 731–738. [Google Scholar] [CrossRef]
- Lee, H.-J.; Im, D.J.; Youn, J.-C.; Chang, S.; Suh, Y.J.; Hong, Y.J.; Kim, Y.J.; Hur, J.; Choi, B.W. Assessment of myocardial delayed enhancement with cardiac computed tomography in cardiomyopathies: A prospective comparison with delayed enhancement cardiac magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2017, 33, 577–584. [Google Scholar] [CrossRef]
- Ohta, Y.; Kitao, S.; Yunaga, H.; Fujii, S.; Mukai, N.; Yamamoto, K.; Ogawa, T. Myocardial delayed enhancement CT for the evaluation of heart failure: Comparison to MRI. Radiology 2018, 288, 682–691. [Google Scholar] [CrossRef]
- Palmisano, A.; Vignale, D.; Benedetti, G.; Del Maschio, A.; De Cobelli, F.; Esposito, A. Late iodine enhancement cardiac computed tomography for detection of myocardial scars: Impact of experience in the clinical practice. Radiol. Med. 2020, 125, 128–136. [Google Scholar] [CrossRef]
- Chang, S.; Han, K.; Youn, J.-C.; Im, D.J.; Kim, J.Y.; Suh, Y.J.; Hong, Y.J.; Hur, J.; Kim, Y.J.; Choi, B.W.; et al. Utility of dual-energy CT-based monochromatic imaging in the assessment of myocardial delayed enhancement in patients with cardiomyopathy. Radiology 2018, 287, 442–451. [Google Scholar] [CrossRef]
- Gould, J.; Sidhu, B.S.; Sieniewicz, B.J.; Porter, B.; Lee, A.W.; Razeghi, O.; Behar, J.M.; Mehta, V.; Elliott, M.K.; Toth, D.; et al. Feasibility of intraprocedural integration of cardiac CT to guide left ventricular lead implantation for CRT upgrades. J. Cardiovasc. Electrophysiol. 2021, 32, 802–812. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.-A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
- Clemente, A.; Seitun, S.; Mantini, C.; Gentile, G.; Federici, D.; Barison, A.; Rossi, A.; Cuman, M.; Pizzuto, A.; Ait-Ali, L.; et al. Cardiac CT angiography: Normal and pathological anatomical features—A narrative review. Cardiovasc. Diagn. Ther. 2020, 10, 1918–1945. [Google Scholar] [CrossRef]
- Booij, R.; Budde, R.P.J.; Dijkshoorn, M.L.; van Straten, M. Technological developments of X-ray computed tomography over half a century: User’s influence on protocol optimization. Eur. J. Radiol. 2020, 131, 109261. [Google Scholar] [CrossRef]
- Manohar, A.; Pack, J.D.; Schluchter, A.J.; McVeigh, E.R. Four-dimensional computed tomography of the left ventricle, Part II: Estimation of mechanical activation times. Med. Phys. 2022, 49, 2309–2323. [Google Scholar] [CrossRef]
- Pack, J.D.; Manohar, A.; Ramani, S.; Claus, B.; Yin, Z.; Contijoch, F.J.; Schluchter, A.J.; McVeigh, E.R. Four-dimensional computed tomography of the left ventricle, Part I: Motion artifact reduction. Med. Phys. 2022, 49, 4404–4418. [Google Scholar] [CrossRef]
- Kędzierski, B.; Macek, P.; Dziadkowiec-Macek, B.; Truszkiewicz, K.; Poręba, R.; Gać, P. Radiation Doses in Cardiovascular Computed Tomography. Life 2023, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Granillo, G.A. Delayed enhancement cardiac computed tomography for the assessment of myocardial infarction: From bench to bedside. Cardiovasc. Diagn. Ther. 2017, 7, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Manohar, A.; Colvert, G.M.; Ortuño, J.E.; Chen, Z.; Yang, J.; Colvert, B.T.; Bandettini, W.P.; Chen, M.Y.; Ledesma-Carbayo, M.J.; McVeigh, E.R. Regional left ventricular endocardial strains estimated from low-dose 4DCT: Comparison with cardiac magnetic resonance feature tracking. Med. Phys. 2022, 49, 5841–5854. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Haase, R.; Schlattmann, P.; Gueret, P.; Andreini, D.; Pontone, G.; Alkadhi, H.; Hausleiter, J.; Leschka, S.; Meijboom, W.B.; Zimmermann, E.; et al. Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: Meta-analysis of individual patient data. BMJ 2019, 365, 1945. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Nagraj, S.; Tzoumas, A.; Arfaras-Melainis, A.; Katamreddy, A.; Sohal, S.; Palaiodimos, L. Diagnostic accuracy of coronary computed tomography angiography in ischemic workup of heart failure: A meta-Analysis. Future Cardiol. 2022, 18, 325–335. [Google Scholar] [CrossRef]
- Andreini, D.; Pontone, G.; Bartorelli, A.L.; Agostoni, P.; Mushtaq, S.; Bertella, E.; Trabattoni, D.; Cattadori, G.; Cortinovis, S.; Annoni, A.; et al. Sixty-four-slice multidetector computed tomography an accurate imaging modality for the evaluation of coronary arteries in dilated cardiomyopathy of unknown etiology. Circ. Cardiovasc. Imaging 2009, 2, 199–205. [Google Scholar] [CrossRef]
- Behar, J.M.; Rajani, R.; Pourmorteza, A.; Preston, R.; Razeghi, O.; Niederer, S.; Adhya, S.; Claridge, S.; Jackson, T.; Sieniewicz, B.; et al. Comprehensive use of cardiac computed tomography to guide left ventricular lead placement in cardiac resynchronization therapy. Heart Rhythm 2017, 14, 1364–1372. [Google Scholar] [CrossRef]
- Truong, Q.A.; Szymonifka, J.; Picard, M.H.; Thai, W.-E.; Wai, B.; Cheung, J.W.; Heist, E.K.; Hoffmann, U.; Singh, J.P. Utility of dual-source computed tomography in cardiac resynchronization therapy—DIRECT study. Heart Rhythm 2018, 15, 1206–1213. [Google Scholar] [CrossRef]
- Worley, S.J.; Gohn, D.C.; Pulliam, R.W. Goose neck snare for LV lead placement in difficult venous anatomy. Pacing Clin. Electrophysiol. 2009, 32, 1577–1581. [Google Scholar] [CrossRef]
- Da Costa, A.; Gate-Martinet, A.; Rouffiange, P.; Cerisier, A.; Nadrouss, A.; Bisch, L.; Romeyer-Bouchard, C.; Isaaz, K. Anatomical factors involved in difficult cardiac resynchronization therapy procedure: A non-invasive study using dual-source 64-multi-slice computed tomography. Europace 2012, 14, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Gach-Kuniewicz, B.; Goncerz, G.; Ali, D.; Kacprzyk, M.; Zarzecki, M.; Loukas, M.; Walocha, J.; Mizia, E. Variations of coronary sinus tributaries among patients undergoing cardiac resynchronization therapy. Folia Morphol. 2022, 82, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, M.R.; Lamb, H.J.; Bax, J.J.; Schuijf, J.D.; de Roos, A.; van der Wall, E.E.; Schalij, M.J. Noninvasive visualization of the cardiac venous system using multislice computed tomography. J. Am. Coll. Cardiol. 2005, 45, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Doganay, S.; Karaman, A.; Gündogdu, F.; Duran, C.; Yalcin, A.; Kantarci, M. Usefulness of multidetector computed tomography coronary venous angiography examination before cardiac resynchronization therapy. Jpn. J. Radiol. 2011, 29, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Van de Veire, N.R.; Marsan, N.A.; Schuijf, J.D.; Bleeker, G.B.; Wijffels, M.C.; van Erven, L.; Holman, E.R.; De Sutter, J.; van der Wall, E.E.; Schalij, M.J.; et al. Noninvasive Imaging of Cardiac Venous Anatomy With 64-Slice Multi-Slice Computed Tomography and Noninvasive Assessment of Left Ventricular Dyssynchrony by 3-Dimensional Tissue Synchronization Imaging in Patients with Heart Failure Scheduled for Cardiac Re. Am. J. Cardiol. 2008, 101, 1023–1029. [Google Scholar] [CrossRef]
- Ricapito, M.d.l.P.; Conde, D.; Theriault, M.M.; Rivera, S.; Badra-Verdu, M.G.; Roux, J.F.; Farand, P.; Ayala-Paredes, F.A.; Gahide, G. Multidetector cardiac tomography: A useful tool before cardiac resynchronization therapy. Cardiol. J. 2015, 22, 590–596. [Google Scholar] [CrossRef]
- Zhou, W.; Hou, X.; Piccinelli, M.; Tang, X.; Tang, L.; Cao, K.; Garcia, E.V.; Zou, J.; Chen, J. 3D Fusion of LV Venous anatomy on fluoroscopy venograms with epicardial surface on SPECT myocardial perfusion images for guiding CRT LV lead placement. JACC Cardiovasc. Imaging 2014, 7, 1239–1248. [Google Scholar] [CrossRef]
- Girsky, M.J.; Shinbane, J.S.; Ahmadi, N.; Mao, S.; Flores, F.; Budoff, M.J. Prospective randomized trial of venous cardiac computed tomographic angiography for facilitation of cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2010, 33, 1182–1187. [Google Scholar] [CrossRef]
- Pezel, T.; Mika, D.; Logeart, D.; Cohen-Solal, A.; Beauvais, F.; Henry, P.; Laissy, J.P.; Moubarak, G. Characterization of non-response to cardiac resynchronization therapy by post-procedural computed tomography. Pacing Clin. Electrophysiol. 2021, 44, 135–144. [Google Scholar] [CrossRef]
- Sun, C.; Pan, Y.; Wang, H.; Li, J.; Nie, P.; Wang, X.; Ma, H.; Huo, F. Assessment of the Coronary Venous System Using 256-Slice Computed Tomography. PLoS ONE 2014, 9, e104246. [Google Scholar] [CrossRef]
- Alikhani, Z.; Li, J.; Merchan, J.A.; Nijhof, N.; Mendel, J.; Orlov, M.V. Coronary sinus anatomy by computerized tomography, overlaid on live fluoroscopy can be successfully used to guide left ventricular lead implantation: A feasibility study. J. Interv. Cardiol. Electrophysiol. 2013, 36, 217–222. [Google Scholar] [CrossRef]
- Młynarski, R.; Młynarska, A.; Gołba, K.S.; Sosnowski, M. Three-dimensional visualisation of coronary sinus ostium from the inside right atrium perspective. Kardiol. Pol. 2018, 76, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Xu, X.; Ji, H.; Liu, J.; Ma, H.; Xie, H.; Dong, J.; Sun, C.; Shi, Y.; Che, K.; et al. Evaluation of the anatomical variations of the coronary venous system in patients with coronary artery calcification using 256-slice computed tomography. PLoS ONE 2020, 15, e0242216. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Kandala, J.; Upadhyay, G.A.; Riedl, L.; Ahmado, I.; Padmanabhan, R.; Gewirtz, H.; Mulligan, L.J.; Singh, J.P. Impact of Myocardial Viability and Left Ventricular Lead Location on Clinical Outcome in Cardiac Resynchronization Therapy Recipients with Ischemic Cardiomyopathy. J. Cardiovasc. Electrophysiol. 2014, 25, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Hessel, S.; Judy, P.; Stein, J.; Abrams, H. Computed tomography of the normal and infarcted myocardium. Am. J. Roentgenol. 1976, 126, 786–791. [Google Scholar] [CrossRef]
- Becker, M.; Zwicker, C.; Kaminski, M.; Napp, A.; Altiok, E.; Ocklenburg, C.; Friedman, Z.; Adam, D.; Schauerte, P.; Marx, N.; et al. Dependency of cardiac resynchronization therapy on myocardial viability at the LV lead position. JACC Cardiovasc. Imaging 2011, 4, 366–374. [Google Scholar] [CrossRef]
- Kydd, A.C.; Khan, F.Z.; Watson, W.D.; Pugh, P.J.; Virdee, M.S.; Dutka, D.P. Prognostic benefit of optimum left ventricular lead position in cardiac resynchronization therapy: Follow-up of the TARGET study cohort (targeted left ventricular lead placement to guide cardiac resynchronization therapy). JACC Heart Fail. 2014, 2, 205–212. [Google Scholar] [CrossRef]
- Fyenbo, D.B.; Sommer, A.; Kühl, J.T.; Kofoed, K.F.; Nørgaard, B.L.; Kronborg, M.B.; Bouchelouche, K.; Nielsen, J.C. Transmural Myocardial Scar Assessed by Cardiac Computed Tomography: Predictor of Echocardiographic Versus Clinical Response to Cardiac Resynchronization Therapy? J. Comput. Assist. Tomogr. 2019, 43, 312–316. [Google Scholar] [CrossRef]
- Hamdy, A.; Kitagawa, K.; Goto, Y.; Yamada, A.; Nakamura, S.; Takafuji, M.; Nagasawa, N.; Sakuma, H. Comparison of the different imaging time points in delayed phase cardiac CT for myocardial scar assessment and extracellular volume fraction estimation in patients with old myocardial infarction. Int. J. Cardiovasc. Imaging 2019, 35, 917–926. [Google Scholar] [CrossRef]
- Kurita, Y.; Kitagawa, K.; Kurobe, Y.; Nakamori, S.; Nakajima, H.; Dohi, K.; Ito, M.; Sakuma, H. Estimation of myocardial extracellular volume fraction with cardiac CT in subjects without clinical coronary artery disease: A feasibility study. J. Cardiovasc. Comput. Tomogr. 2016, 10, 237–241. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Schoepf, U.J.; van Assen, M.; Alimohamed, I.; Griffith, L.P.; Luo, T.; Sun, Z.; Fan, Z.; Xu, L. Extracellular volume quantitation using dual-energy CT in patients with heart failure: Comparison with 3T cardiac MR. Int. J. Cardiol. 2018, 268, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Im, D.J.; Youn, J.-C.; Chang, S.; Suh, Y.J.; Hong, Y.J.; Kim, Y.J.; Hur, J.; Choi, B.W. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-Enhanced cardiac ct in nonischemic cardiomyopathy: A prospective comparison with cardiac MR imaging. Radiology 2016, 280, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lin, L.; Xu, C.; Han, Y.; Lin, X.; Hou, Y.; Lu, X.; Vembar, M.; Jin, Z.; Wang, Y. Quantitative analysis of late iodine enhancement using dual-layer spectral detector computed tomography: Comparison with magnetic resonance imaging. Quant. Imaging Med. Surg. 2022, 12, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, H.; Xie, J.; Yu, H.; Chen, W.; Yin, K.; Chen, X.; Sheng, Z.; Zhang, X.; Mu, D. Iodine-based extracellular volume for evaluating myocardial status in patients undergoing percutaneous coronary intervention for acute myocardial infarction by using dual-layer spectral detector computed tomography: A comparison study with magnetic resona. Quant. Imaging Med. Surg. 2022, 12, 4502–4511. [Google Scholar] [CrossRef]
- Baggiano, A.; Conte, E.; Spiritigliozzi, L.; Mushtaq, S.; Annoni, A.; Carerj, M.L.; Cilia, F.; Fazzari, F.; Formenti, A.; Frappampina, A.; et al. Quantification of extracellular volume with cardiac computed tomography in patients with dilated cardiomyopathy. J. Cardiovasc. Comput. Tomogr. 2023, 17, 261–268. [Google Scholar] [CrossRef]
- Dubourg, B.; Dacher, J.-N.; Durand, E.; Caudron, J.; Bauer, F.; Bubenheim, M.; Eltchaninoff, H.; Serfaty, J.-M. Single-source dual energy CT to assess myocardial extracellular volume fraction in aortic stenosis before transcatheter aortic valve implantation (TAVI). Diagn. Interv. Imaging 2021, 102, 561–570. [Google Scholar] [CrossRef]
- Tavoosi, A.; Brito, J.B.d.O.; El Mais, H.; Small, T.D.; Crean, A.M.; Chow, B.J.; Small, G.R. Dual versus single energy cardiac CT to measure extra cellular volume in cardiac amyloidosis: Correlations with cardiac MRI. IJC Heart Vasc. 2023, 44, 101166. [Google Scholar] [CrossRef]
- Oda, S.; Emoto, T.; Nakaura, T.; Kidoh, M.; Utsunomiya, D.; Funama, Y.; Nagayama, Y.; Takashio, S.; Ueda, M.; Yamashita, T.; et al. Myocardial Late Iodine Enhancement and Extracellular Volume Quantification with Dual-Layer Spectral Detector Dual-Energy Cardiac CT. Radiol. Cardiothorac. Imaging 2019, 1, e180003. [Google Scholar] [CrossRef]
- Kim, N.Y.; Im, D.J.; Youn, J.-C.; Hong, Y.J.; Choi, B.W.; Kang, S.-M.; Lee, H.-J. Synthetic Extracellular Volume Fraction Derived Using Virtual Unenhanced Attenuation of Blood on Contrast-Enhanced Cardiac Dual-Energy CT in Nonischemic Cardiomyopathy. Am. J. Roentgenol. 2022, 218, 454–461. [Google Scholar] [CrossRef]
- Qi, R.-X.; Shao, J.; Jiang, J.-S.; Ruan, X.-W.; Huang, S.; Zhang, Q.; Hu, C.-H. Myocardial extracellular volume fraction quantitation using cardiac dual-energy CT with late iodine enhancement in patients with heart failure without coronary artery disease: A single-center prospective study. Eur. J. Radiol. 2021, 140, 109743. [Google Scholar] [CrossRef]
- Qi, R.-X.; Jiang, J.-S.; Shao, J.; Zhang, Q.; Zheng, K.-L.; Xiao, J.; Huang, S.; Gong, S.-C. Measurement of myocardial extracellular volume fraction in patients with heart failure with preserved ejection fraction using dual-energy computed tomography. Eur. Radiol. 2022, 32, 4253–4263. [Google Scholar] [CrossRef] [PubMed]
- Abadia, A.F.; van Assen, M.; Martin, S.S.; Vingiani, V.; Griffith, L.P.; Giovagnoli, D.A.; Bauer, M.J.; Schoepf, U.J. Myocardial extracellular volume fraction to differentiate healthy from cardiomyopathic myocardium using dual-source dual-energy CT. J. Cardiovasc. Comput. Tomogr. 2020, 14, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Abadia, A.F.; Aquino, G.J.; Schoepf, U.J.; Wels, M.; Schmidt, B.; Sahbaee, P.; Dargis, D.M.B.; Burt, J.R.; Varga-Szemes, A.; Emrich, T. Automated Dual-energy Computed Tomography-based Extracellular Volume Estimation for Myocardial Characterization in Patients with Ischemic and Nonischemic Cardiomyopathy. J. Thorac. Imaging 2022, 37, 307–314. [Google Scholar] [CrossRef]
- van Assen, M.; De Cecco, C.N.; Sahbaee, P.; Eid, M.H.; Griffith, L.P.; Bauer, M.J.; Savage, R.H.; Varga-Szemes, A.; Oudkerk, M.; Vliegenthart, R.; et al. Feasibility of extracellular volume quantification using dual-energy CT. J. Cardiovasc. Comput. Tomogr. 2019, 13, 81–84. [Google Scholar] [CrossRef]
- Gama, F.; Rosmini, S.; Bandula, S.; Patel, K.P.; Massa, P.; Tobon-Gomez, C.; Ecke, K.; Stroud, T.; Condron, M.; Thornton, G.D.; et al. Extracellular Volume Fraction by Computed Tomography Predicts Long-Term Prognosis Among Patients with Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2022, 15, 2082–2094. [Google Scholar] [CrossRef]
- Wang, R.; Fang, Z.; Wang, H.; Schoepf, U.J.; Emrich, T.; Giovagnoli, D.; Biles, E.; Zhou, Z.; Du, Z.; Liu, T.; et al. Quantitative analysis of three-dimensional left ventricular global strain using coronary computed tomography angiography in patients with heart failure: Comparison with 3T cardiac MR. Eur. J. Radiol. 2021, 135, 109485. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, B.; Grogg, H.; Zurkirchen, J.; Demirel, C.; Hagemeyer, D.; Okuno, T.; Brugger, N.; De Marchi, S.; Huber, A.T.; Berto, M.B.; et al. Reproducibility of 4D cardiac computed tomography feature tracking myocardial strain and comparison against speckle-tracking echocardiography in patients with severe aortic stenosis. J. Cardiovasc. Comput. Tomogr. 2022, 16, 309–318. [Google Scholar] [CrossRef]
- Fukui, M.; Xu, J.; Abdelkarim, I.; Sharbaugh, M.S.; Thoma, F.W.; Althouse, A.D.; Pedrizzetti, G.; Cavalcante, J.L. Global longitudinal strain assessment by computed tomography in severe aortic stenosis patients—Feasibility using feature tracking analysis. J. Cardiovasc. Comput. Tomogr. 2019, 13, 157–162. [Google Scholar] [CrossRef]
- Marwan, M.; Ammon, F.; Bittner, D.; Röther, J.; Mekkhala, N.; Hell, M.; Schuhbaeck, A.; Gitsioudis, G.; Feyrer, R.; Schlundt, C.; et al. CT-derived left ventricular global strain in aortic valve stenosis patients: A comparative analysis pre and post transcatheter aortic valve implantation. J. Cardiovasc. Comput. Tomogr. 2018, 12, 240–244. [Google Scholar] [CrossRef]
- Fukui, M.; Xu, J.; Thoma, F.; Sultan, I.; Mulukutla, S.; Elzomor, H.; Lee, J.S.; Gleason, T.G.; Cavalcante, J.L. Baseline global longitudinal strain by computed tomography is associated with post transcatheter aortic valve replacement outcomes. J. Cardiovasc. Comput. Tomogr. 2020, 14, 233–239. [Google Scholar] [CrossRef]
- Gegenava, T.; van der Bijl, P.; Vollema, E.M.; van der Kley, F.; de Weger, A.; Hautemann, D.; Reiber, J.H.; Marsan, N.A.; Bax, J.J.; Delgado, V. Prognostic Influence of Feature Tracking Multidetector Row Computed Tomography-Derived Left Ventricular Global Longitudinal Strain in Patients with Aortic Stenosis Treated with Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 125, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Appadurai, V.; D’Elia, N.; Mew, T.; Tomlinson, S.; Chan, J.; Hamilton-Craig, C.; Scalia, G.M. Global longitudinal strain as a prognostic marker in cardiac resynchronisation therapy: A systematic review. IJC Heart Vasc. 2021, 35, 100849. [Google Scholar] [CrossRef] [PubMed]
- Wouters, P.C.; van Slochteren, F.J.; Tuinenburg, A.E.; Doevendans, P.A.; Cramer, M.-J.M.; Delnoy, P.-P.H.; van Dijk, V.F.; Meine, M. On-screen image-guided lead placement in cardiac resynchronization therapy: Feasibility and outcome in a multicenter setting. Heart Rhythm O2 2023, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Kido, T.; Kurata, A.; Sawada, S.; Suekuni, H.; Kido, T.; Yokoi, T.; Uetani, T.; Inoue, K.; Miyagawa, M.; et al. Three-dimensional maximum principal strain using cardiac computed tomography for identification of myocardial infarction. Eur. Radiol. 2017, 27, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Shiina, Y.; Inai, K.; Takahashi, T.; Shimomiya, Y.; Nagao, M. Clinical impact of cardiac computed tomography derived three-dimensional strain for adult congenital heart disease: A pilot study. Int. J. Cardiovasc. Imaging 2020, 36, 131–140. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, L.; Zhang, M.C.; Sun, H.; Zeng, H.; Yang, P. Imaging of Pericardiophrenic Bundles Using Multislice Spiral Computed Tomography for Phrenic Nerve Anatomy. J. Cardiovasc. Electrophysiol. 2016, 27, 961–971. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Krishnan, S.; Fowler, S.J.; Saremi, F.; Kondo, T.; Ahsan, C.; Narula, J.; Gurudevan, S. Detection of Phrenic Nerves and Their Relation to Cardiac Anatomy Using 64-Slice Multidetector Computed Tomography. Am. J. Cardiol. 2007, 100, 133–137. [Google Scholar] [CrossRef]
- Duckett, S.G.; Ginks, M.R.; Knowles, B.R.; Ma, Y.; Shetty, A.; Bostock, J.; Cooklin, M.; Gill, J.S.; Carr-White, G.S.; Razavi, R.; et al. Advanced Image Fusion to Overlay Coronary Sinus Anatomy with Real-Time Fluoroscopy to Facilitate Left Ventricular Lead Implantation in CRT. Pacing Clin. Electrophysiol. 2011, 34, 226–234. [Google Scholar] [CrossRef]
- Manohar, A.; Colvert, G.M.; Yang, J.; Chen, Z.; Ledesma-Carbayo, M.J.; Kronborg, M.B.; Sommer, A.; Nørgaard, B.L.; Nielsen, J.C.; McVeigh, E.R. Prediction of Cardiac Resynchronization Therapy Response Using a Lead Placement Score Derived from 4-Dimensional Computed Tomography. Circ. Cardiovasc. Imaging 2022, 15, E014165. [Google Scholar] [CrossRef]
- Hadwiger, M.; Schumann, L.; Eisemann, N.; Dagres, N.; Hindricks, G.; Haug, J.; Wolf, M.; Marschall, U.; Katalinic, A.; Frielitz, F.-S. A long-term cost-effectiveness analysis of cardiac resynchronisation therapy with or without defibrillator based on health claims data. Cost Eff. Resour. Alloc. 2022, 20, 48. [Google Scholar] [CrossRef]
- Wouters, P.C.; van Lieshout, C.; van Dijk, V.F.; Delnoy, P.-P.H.; Doevendans, P.A.; Cramer, M.J.; Frederix, G.W.; van Slochteren, F.J.; Meine, M. Advanced image-supported lead placement in cardiac resynchronisation therapy: Protocol for the multicentre, randomised controlled ADVISE trial and early economic evaluation. BMJ Open 2021, 11, e054115. [Google Scholar] [CrossRef] [PubMed]
- Biloglav, Z.; Medaković, P.; Buljević, J.; Žuvela, F.; Padjen, I.; Vrkić, D.; Ćurić, J. The analysis of waiting time and utilization of computed tomography and magnetic resonance imaging in Croatia: A nationwide survey. Croat. Med. J. 2020, 61, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T.J.; Deseive, S.; Leipsic, J.; Hadamitzky, M.; Chen, M.Y.; Rubinshtein, R.; Heckner, M.; Bax, J.J.; Fang, X.-M.; Grove, E.L.; et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: Results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy in daily practice in 2017 (PROTECTION VI). Eur. Heart J. 2018, 39, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.-H.; Chen, L.-J.; Hu, L.-W.; Ouyang, R.-Z.; Guo, C.; Sun, A.-M.; Wang, Q.; Qiu, H.-S.; Yan, Q.; Zhang, Y.-Q.; et al. Postoperative evaluation of left ventricular global strain using cardiac computed tomography in pediatric patients with congenital heart disease: A comparison with echocardiography. Eur. J. Radiol. 2021, 142, e109868. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Ahlman, M.A.; Mallek, M.; Cork, T.E.; Chen, M.Y.; Bluemke, D.A.; Sandfort, V. Cardiac cine CT approaching 1 mSv: Implementation and assessment of a 58-ms temporal resolution protocol. Int. J. Cardiovasc. Imaging 2020, 36, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, M.; Kitagawa, K.; Nakamura, S.; Hamdy, A.; Goto, Y.; Ishida, M.; Sakuma, H. Feasibility of extracellular volume fraction calculation using myocardial CT delayed enhancement with low contrast media administration. J. Cardiovasc. Comput. Tomogr. 2020, 14, 524–528. [Google Scholar] [CrossRef]
- Jin, C.; Dai, Q.; Li, P.; Lam, P.; Cha, Y.-M. Left bundle branch area pacing for heart failure patients requiring cardiac resynchronization therapy: A meta-analysis. J. Cardiovasc. Electrophysiol. 2023, 34, 1933–1943. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Zalavadia, D.; Haseeb, A.; Dye, C.; Madan, N.; Skeete, J.R.; Vipparthy, S.C.; Young, W.; Ravi, V.; Rajakumar, C.; et al. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm 2022, 19, 1263–1271. [Google Scholar] [CrossRef]




| CT Study | Purpose |
|---|---|
| Cardiac | Left ventricular dimension and function: baseline (and follow-up) |
| Coronary | Coronary artery and venous system anatomy and patency |
| Late phase | Myocardial fibrosis delineation (LIE) and extracellular volume (ECV) |
| Strain * | Mechanical dyssynchrony |
| Patient Sub-Type with an Indication for CRT | Class Recommendation |
|---|---|
| Left ventricular ejection fraction < 35% with a left bundle branch block with a QRS duration of: | |
| • More than 150 ms | I |
| • 130–149 ms | IIa |
| Left ventricular ejection fraction < 35% with a non-left bundle branch block with a duration of: | |
| • More than 150 ms | IIa |
| • 130–149 ms | IIb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerrits, W.; Danad, I.; Velthuis, B.; Mushtaq, S.; Cramer, M.J.; van der Harst, P.; van Slochteren, F.J.; Meine, M.; Suchá, D.; Guglielmo, M. Cardiac CT in CRT as a Singular Imaging Modality for Diagnosis and Patient-Tailored Management. J. Clin. Med. 2023, 12, 6212. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12196212
Gerrits W, Danad I, Velthuis B, Mushtaq S, Cramer MJ, van der Harst P, van Slochteren FJ, Meine M, Suchá D, Guglielmo M. Cardiac CT in CRT as a Singular Imaging Modality for Diagnosis and Patient-Tailored Management. Journal of Clinical Medicine. 2023; 12(19):6212. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12196212
Chicago/Turabian StyleGerrits, Willem, Ibrahim Danad, Birgitta Velthuis, Saima Mushtaq, Maarten J. Cramer, Pim van der Harst, Frebus J. van Slochteren, Mathias Meine, Dominika Suchá, and Marco Guglielmo. 2023. "Cardiac CT in CRT as a Singular Imaging Modality for Diagnosis and Patient-Tailored Management" Journal of Clinical Medicine 12, no. 19: 6212. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm12196212