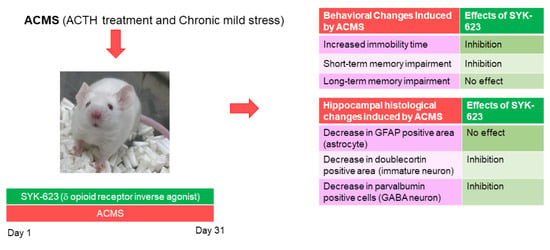
SYK-623, a δ Opioid Receptor Inverse Agonist, Mitigates Chronic Stress-Induced Behavioral Abnormalities and Disrupted Neurogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. ACMS Model Mice
2.3. Drugs
2.4. Tail Suspension Test
2.5. Spontaneous Alternation Test (Y-Maze)
2.6. Novel Arm Recognition Test (Modified Y-Maze)
2.7. Novel Object Recognition Tests
2.8. Open Field Test
2.9. Immunohistochemistry
2.10. SDS-PAGE and Western Blotting
2.11. Statistics
3. Results
3.1. ACMS-Induced Imipramine-Resistant Physical and Behavioral Impairments
3.2. DOR Inverse Agonist SYK-623 Prevented Induction of Behavioral Impairment in ACMS Mice
3.3. The DOR Neutral Antagonist NTI Did Not Suppress ACMS-Induced Behavioral Impairment
3.4. SYK-623 Does Not Suppress ACMS-Induced Imipramine-Resistant Astrocyte Loss in the Hippocampus
3.5. SYK-623 Suppressed ACMS-Induced Imipramine-Resistant Decrease in Neurogenesis
3.6. SYK-623 Suppresses ACMS-Induced Impairment of GABA Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richter-Levin, G.; Xu, L. How could stress lead to major depressive disorder? IBRO Rep. 2018, 4, 38–43. [Google Scholar] [CrossRef]
- Brunson, K.L.; Kramár, E.; Lin, B.; Chen, Y.; Colgin, L.L.; Yanagihara, T.K.; Lynch, G.; Baram, T.Z. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005, 25, 9328–9338. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Alonso, A.; McClure, L.A.; Hajjar, I.; Manly, J.J.; Judd, S. Association of stress with cognitive function among older black and white US adults. JAMA Netw. Open 2023, 6, e231860. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, J.J. Neurocognitive effects of stress: A metaparadigm perspective. Mol. Psychiatry 2023, 28, 2750–2763. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Liu, Y.; Kiselev, D.; Khairuddin, S.; Chiu, J.L.Y.; Lam, J.; Chan, Y.-S.; Pavlov, D.; Proshin, A.; Lesch, K.-P.; et al. Chronic mild stress paradigm as a rat model of depression: Facts, artifacts, and future perspectives. Psychopharmacology 2022, 239, 663–693. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Brignone, M.; Larsen, K.G. A network meta-analysis comparing effects of various antidepressant classes on the Digit Symbol Substitution Test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int. J. Neuropsychopharmacol. 2018, 21, 97–107. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, D.; Li, S.; Li, G.; Shyamala, S.G.; Barish, P.A.; Vernon, M.M.; Pan, J.; Ogle, W.O. Curcumin reverses impaired cognition and neuronal plasticity induced by chronic stress. Neuropharmacology 2009, 57, 463–471. [Google Scholar] [CrossRef]
- Song, L.; Che, W.; Min-Wei, W.; Murakami, Y.; Matsumoto, K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol. Biochem. Behav. 2006, 83, 186–193. [Google Scholar] [CrossRef]
- Elizalde, N.; Gil-Bea, F.J.; Ramírez, M.J.; Aisa, B.; Lasheras, B.; Del Rio, J.; Tordera, R.M. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: Effect of antidepressant treatment. Psychopharmacology 2008, 199, 1–14. [Google Scholar] [CrossRef]
- Orsetti, M.; Colella, L.; Dellarole, A.; Canonico, P.L.; Ghi, P. Modification of spatial recognition memory and object discrimination after chronic administration of haloperidol, amitriptyline, sodium valproate or olanzapine in normal and anhedonic rats. Int. J. Neuropsychopharmacol. 2007, 10, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Erbs, E.; Faget, L.; Scherrer, G.; Matifas, A.; Filliol, D.; Vonesch, J.-L.; Koch, M.; Kessler, P.; Hentsch, D.; Birling, M.-C.; et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct. Funct. 2015, 220, 677–702. [Google Scholar] [CrossRef]
- Saitoh, A.; Yamada, M. Antidepressant-like effects of δ opioid receptor agonists in animal models. Curr. Neuropharmacol. 2012, 10, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.A.; Hoshaw, B.A.; Unterwald, E.M. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br. J. Pharmacol. 2006, 147, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Kamei, J. Delta-opioid receptor antagonists as a new concept for central acting antitussive drugs. Pulm. Pharmacol. Ther. 2002, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Iwai, T.; Higashi, E.; Nakamura, M.; Iwamatsu, C.; Itoh, K.; Nemoto, T.; Tanabe, M.; Fujii, H. Discovery of δ opioid receptor full inverse agonists and their effects on restraint stress-induced cognitive impairment in mice. ACS Chem. Neurosci. 2019, 10, 2237–2242. [Google Scholar] [CrossRef]
- Higashi, E.; Hirayama, S.; Nikaido, J.; Shibasaki, M.; Kono, T.; Honjo, A.; Ikeda, H.; Kamei, J.; Fujii, H. Development of novel δ opioid receptor inverse agonists without a basic nitrogen atom and their antitussive effects in mice. ACS Chem. Neurosci. 2019, 10, 3939–3945. [Google Scholar] [CrossRef]
- Berg, K.A.; Clarke, W.P. Making Sense of Pharmacology: Inverse agonism and functional selectivity. Int. J. Neuropsychopharmacol. 2018, 21, 962–977. [Google Scholar] [CrossRef]
- Iwai, T.; Okonogi, T.; Mishima, R.; Yoshida, K.; Oyama, M.; Watanabe, S.; Hirayama, S.; Fujii, H.; Tanabe, M. δ-Opioid receptor inverse agonist SYK-623 improved stress-induced learning dysfunction in mice. In Proceedings of the Annual Meeting of The Japanese Pharmacological Society WCP2018 (The 18th World Congress of Basic and Clinical Pharmacology), Kyoto, Japan, 1–6 July 2018; p. PO1-1–20. [Google Scholar] [CrossRef]
- Doi, M.; Miyazaki, I.; Nagamachi, T.; Shinomiya, K.; Matsunaga, H.; Sendo, T.; Kawasaki, H.; Asanuma, M.; Gomita, Y.; Kitamura, Y. Effects of imipramine and lithium on the suppression of cell proliferation in the dentate gyrus of the hippocampus in adrenocorticotropic hormone-treated rats. Acta Med. Okayama 2010, 64, 219–223. [Google Scholar]
- Iwai, T.; Ohnuki, T.; Sasaki-Hamada, S.; Saitoh, A.; Sugiyama, A.; Oka, J.-I. Glucagon-like peptide-2 but not imipramine exhibits antidepressant-like effects in ACTH-treated mice. Behav. Brain Res. 2013, 243, 153–157. [Google Scholar] [CrossRef]
- Sanacora, G.; Banasr, M. From pathophysiology to novel antidepressant drugs: Glial contributions to the pathology and treatment of mood disorders. Biol. Psychiatry 2013, 73, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Darcet, F.; Gardier, A.M.; Gaillard, R.; David, D.J.; Guilloux, J.-P. Cognitive dysfunction in major depressive disorder. A translational review in animal models of the disease. Pharmaceuticals 2016, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, F.; Amin, N.; Ren, Q.; Ye, S.; Hu, Z.; Tan, X.; Jiang, M.; Fang, M. Defects of parvalbumin-positive interneurons in the ventral dentate gyrus region are implicated depression-like behavior in mice. Brain Behav. Immun. 2022, 99, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Reliability of the chronic mild stress model of depression: A user survey. Neurobiol. Stress 2017, 6, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Hayashi, Y.; Narita, S.; Kasuya, Y.; Jin, K.; Tsugane, M.; Oka, J.-I. Antidepressant-like effects of glucagon-like peptide-2 in mice occur via monoamine pathways. Behav. Brain Res. 2009, 204, 235–240. [Google Scholar] [CrossRef]
- Iwai, T.; Kikuchi, A.; Oyama, M.; Watanabe, S.; Tanabe, M. Mirogabalin prevents repeated restraint stress-induced dysfunction in mice. Behav. Brain Res. 2020, 383, 112506. [Google Scholar] [CrossRef]
- Zhao, Q.; Niu, Y.; Matsumoto, K.; Tsuneyama, K.; Tanaka, K.; Miyata, T.; Yokozawa, T. Chotosan ameliorates cognitive and emotional deficits in an animal model of type 2 diabetes: Possible Involvement of cholinergic and VEGF/PDGF mechanisms in the brain. BMC Complement Altern. Med. 2012, 12, 188. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ludbrook, J. Multiple Comparison Procedures Updated. Clin. Exp. Pharmacol. Physiol. 1998, 25, 1032–1037. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Figueiredo, H.F.; Ostrander, M.M.; Choi, D.C.; Engeland, W.C.; Herman, J.P. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E965–E973. [Google Scholar] [CrossRef] [PubMed]
- Machado-Santos, A.R.; Loureiro-Campos, E.; Patrício, P.; Araújo, B.; Alves, N.D.; Mateus-Pinheiro, A.; Correia, J.S.; Morais, M.; Bessa, J.M.; Sousa, N.; et al. Beyond new neurons in the adult hippocampus: Imipramine acts as a pro-astrogliogenic factor and rescues cognitive impairments induced by stress exposure. Cells 2022, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Christian, K.M.; Ming, G.; Song, H. Modification of hippocampal circuitry by adult neurogenesis. Dev. Neurobiol. 2012, 72, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Huang, H.; Zhang, Y.; Lv, J.; Wang, Q.; He, Q.; Liu, X. Ginsenoside Rb1 produces antidepressant-like effects in a chronic social defeat stress model of depression through the BDNF-TrkB signaling pathway. Front. Pharmacol. 2021, 12, 680903. [Google Scholar] [CrossRef] [PubMed]
- Podolan, M.; Dos Santos, J.; Walber, T.; Possamai, F.; Viola, G.G.; Lino De Oliveira, C. A single injection of imipramine affected proliferation in the hippocampus of adult swiss mice depending on the route of administration, doses, survival time and lodging conditions. J. Chem. Neuroanat. 2019, 100, 101655. [Google Scholar] [CrossRef]
- Van Bokhoven, P.; Oomen, C.A.; Hoogendijk, W.J.G.; Smit, A.B.; Lucassen, P.J.; Spijker, S. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment: Lasting effect of social stress on neurogenesis. Eur. J. Neurosci. 2011, 33, 1833–1840. [Google Scholar] [CrossRef]
- Perez, S.M.; Boley, A.; Lodge, D.J. Region specific knockdown of parvalbumin or somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Transl. Psychiatry 2019, 9, 264. [Google Scholar] [CrossRef]
- Perlman, G.; Tanti, A.; Mechawar, N. Parvalbumin interneuron alterations in stress-related mood disorders: A systematic review. Neurobiol. Stress 2021, 15, 100380. [Google Scholar] [CrossRef]
- Bohmbach, K.; Henneberger, C.; Hirrlinger, J. Astrocytes in memory formation and maintenance. Essays Biochem. 2023, 67, 107–117. [Google Scholar] [CrossRef]
- Lee, H.S.; Ghetti, A.; Pinto-Duarte, A.; Wang, X.; Dziewczapolski, G.; Galimi, F.; Huitron-Resendiz, S.; Piña-Crespo, J.C.; Roberts, A.J.; Verma, I.M.; et al. Astrocytes contribute to gamma oscillations and recognition memory. Proc. Natl. Acad. Sci. USA 2014, 111, E3343–E3352. [Google Scholar] [CrossRef]
- Pan, Y.-W.; Storm, D.R.; Xia, Z. Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: New insights from ERK5 MAP kinase. Neurobiol. Learn. Mem. 2013, 105, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.D.; Duman, R.S. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. 2007, 18, 391–418. [Google Scholar] [CrossRef]
- Diniz, L.; Dos Santos, T.B.; Britto, L.R.G.; Céspedes, I.C.; Garcia, M.C.; Spadari-Bratfisch, R.C.; Medalha, C.C.; De Castro, G.M.; Montesano, F.T.; Viana, M.B. Effects of chronic treatment with corticosterone and imipramine on Fos immunoreactivity and adult hippocampal neurogenesis. Behav. Brain Res. 2013, 238, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Surget, A.; Saxe, M.; Leman, S.; Ibarguen-Vargas, Y.; Chalon, S.; Griebel, G.; Hen, R.; Belzung, C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry 2008, 64, 293–301. [Google Scholar] [CrossRef] [PubMed]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.-W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.-P.; et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef]
- Kitamura, Y.; Doi, M.; Kuwatsuka, K.; Onoue, Y.; Miyazaki, I.; Shinomiya, K.; Koyama, T.; Sendo, T.; Kawasaki, H.; Asanuma, M.; et al. Chronic treatment with imipramine and lithium increases cell proliferation in the hippocampus in adrenocorticotropic hormone-treated rats. Biol. Pharm. Bull. 2011, 34, 77–81. [Google Scholar] [CrossRef]
- Kitamura, Y.; Araki, H.; Suemaru, K.; Gomita, Y. Effects of imipramine and lithium on wet-dog shakes mediated by the 5-HT2A receptor in ACTH-treated rats. Pharmacol. Biochem. Behav. 2002, 72, 397–402. [Google Scholar] [CrossRef]
- Klempin, F.; Babu, H.; De Pietri Tonelli, D.; Alarcon, E.; Fabel, K.; Kempermann, G. Oppositional Effects of Serotonin Receptors 5-HT1a, 2, and 2c in the Regulation of Adult Hippocampal Neurogenesis. Front. Mol. Neurosci. 2010, 3, 14. [Google Scholar] [CrossRef]
- Erbs, E.; Faget, L.; Scherrer, G.; Kessler, P.; Hentsch, D.; Vonesch, J.-L.; Matifas, A.; Kieffer, B.L.; Massotte, D. Distribution of delta opioid receptor-expressing neurons in the mouse hippocampus. Neuroscience 2012, 221, 203–213. [Google Scholar] [CrossRef]
- Korotkova, T.; Fuchs, E.C.; Ponomarenko, A.; Von Engelhardt, J.; Monyer, H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 2010, 68, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Morellini, F.; Sivukhina, E.; Stoenica, L.; Oulianova, E.; Bukalo, O.; Jakovcevski, I.; Dityatev, A.; Irintchev, A.; Schachner, M. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb. Cortex 2010, 20, 2712–2727. [Google Scholar] [CrossRef] [PubMed]
- Catavero, C.; Bao, H.; Song, J. Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res. 2018, 371, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, J.; Moss, J.; Wen, Z.; Sun, G.J.; Hsu, D.; Zhong, C.; Davoudi, H.; Christian, K.M.; Toni, N.; et al. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat. Neurosci. 2013, 16, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rabaza, V.; Llorens-Martín, M.; Velázquez-Sánchez, C.; Ferragud, A.; Arcusa, A.; Gumus, H.G.; Gómez-Pinedo, U.; Pérez-Villalba, A.; Roselló, J.; Trejo, J.L.; et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience 2009, 159, 59–68. [Google Scholar] [CrossRef]
- Saxe, M.D.; Malleret, G.; Vronskaya, S.; Mendez, I.; Garcia, A.D.; Sofroniew, M.V.; Kandel, E.R.; Hen, R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl. Acad. Sci. USA 2007, 104, 4642–4646. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.D.; Correia, J.S.; Patrício, P.; Mateus-Pinheiro, A.; Machado-Santos, A.R.; Loureiro-Campos, E.; Morais, M.; Bessa, J.M.; Sousa, N.; Pinto, L. Adult hippocampal neuroplasticity triggers susceptibility to recurrent depression. Transl. Psychiatry 2017, 7, e1058. [Google Scholar] [CrossRef]
- Foyet, H.S.; Tchinda Deffo, S.; Koagne Yewo, P.; Antioch, I.; Zingue, S.; Asongalem, E.A.; Kamtchouing, P.; Ciobica, A. Ficus sycomorus extract reversed behavioral impairment and brain oxidative stress induced by unpredictable chronic mild stress in rats. BMC Complement Altern. Med. 2017, 17, 502. [Google Scholar] [CrossRef]
- Matcham, F.; Simblett, S.K.; Leightley, D.; Dalby, M.; Siddi, S.; Haro, J.M.; Lamers, F.; Penninx, B.W.H.J.; Bruce, S.; Nica, R.; et al. The association between persistent cognitive difficulties and depression and functional outcomes in people with major depressive disorder. Psychol. Med. 2023, 53, 6334–6344. [Google Scholar] [CrossRef]
- De Diego-Adeliño, J.; Crespo, J.M.; Mora, F.; Neyra, A.; Iborra, P.; Gutiérrez-Rojas, L.; Salonia, S.F. Vortioxetine in major depressive disorder: From mechanisms of action to clinical studies. An Updated Review. Expert Opin. Drug Saf. 2022, 21, 673–690. [Google Scholar] [CrossRef]
- Gärtner, M.; Ghisu, M.E.; Scheidegger, M.; Bönke, L.; Fan, Y.; Stippl, A.; Herrera-Melendez, A.-L.; Metz, S.; Winnebeck, E.; Fissler, M.; et al. Aberrant working memory processing in major depression: Evidence from multivoxel pattern classification. Neuropsychopharmacology 2018, 43, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, A.; Yamada, M.; Yamada, M.; Takahashi, K.; Yamaguchi, K.; Murasawa, H.; Nakatani, A.; Tatsumi, Y.; Hirose, N.; Kamei, J. Antidepressant-like effects of the delta-opioid receptor agonist SNC80 ([(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in an olfactory bulbectomized rat model. Brain Res. 2008, 1208, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, L.; Saitoh, A.; Yamada, M.; Fujii, H.; Nagase, H.; Yamada, M. Effects of repeated treatment with a delta opioid receptor agonist KNT-127 on hyperemotionality in olfactory-bulbectomized rats. Behav. Brain Res. 2017, 323, 11–14. [Google Scholar] [CrossRef]
- Yoshioka, T.; Yamada, D.; Segi-Nishida, E.; Nagase, H.; Saitoh, A. KNT-127, a selective delta opioid receptor agonist, shows beneficial effects in the hippocampal dentate gyrus of a chronic vicarious social defeat stress mouse model. Neuropharmacology 2023, 232, 109511. [Google Scholar] [CrossRef]
- Husain, S.; Ahmad, A.; Singh, S.; Peterseim, C.; Abdul, Y.; Nutaitis, M.J. PI3K/Akt Pathway: A role in δ-opioid receptor-mediated RGC neuroprotection. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Tanguturi, P.; Pathak, V.; Zhang, S.; Moukha-Chafiq, O.; Augelli-Szafran, C.E.; Streicher, J.M. Discovery of novel delta opioid receptor (DOR) inverse agonist and irreversible (non-competitive) antagonists. Molecules 2021, 26, 6693. [Google Scholar] [CrossRef] [PubMed]
- Khakha, N.; Khan, H.; Kaur, A.; Singh, T.G. Therapeutic implications of phosphorylation- and dephosphorylation-dependent factors of cAMP-response element-binding protein (CREB) in neurodegeneration. Pharmacol. Rep. 2023, 75, 1152–1165. [Google Scholar] [CrossRef]
- Hebb, A.L.O.; Robertson, H.A. Role of phosphodiesterases in neurological and psychiatric disease. Curr. Opin. Pharmacol. 2007, 7, 86–92. [Google Scholar] [CrossRef]
- Bergantin, L.B.; Caricati-Neto, A. challenges for the pharmacological treatment of neurological and psychiatric disorders: Implications of the Ca(2+)/cAMP intracellular signaling interaction. Eur. J. Pharmacol. 2016, 788, 255–260. [Google Scholar] [CrossRef]
- Kawaminami, A.; Yamada, D.; Yanagisawa, S.; Shirakata, M.; Iio, K.; Nagase, H.; Saitoh, A. Selective δ-opioid receptor agonist, KNT-127, facilitates contextual fear extinction via infralimbic cortex and amygdala in mice. Front. Behav. Neurosci. 2022, 16, 808232. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Li, Y.; Chen, S.; Yang, X.-F.; Min, J.-W. Biphalin, a dimeric opioid peptide, reduces neonatal hypoxia-ischemia brain injury in mice by the activation of PI3K/Akt signaling pathway. J. Chem. Neuroanat. 2021, 115, 101967. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, R.; Zhi, F.; Sheng, S.; Khiati, L.; Yang, Y.; Peng, Y.; Xia, Y. δ-Opioid receptor, microglia and neuroinflammation. Aging Dis. 2023, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef] [PubMed]






| Days | Drug Treatment | Short Stressor (9:30–14:00) | Overnight Stressor |
|---|---|---|---|
| 1 | ACTH + Vehicle, Imipramine, SYK-623, or NTI | Restraint stress | Night lighting |
| 2 | Forced swimming | Cage tilting | |
| 3 | Restraint stress | Bed deprivation | |
| 4 | Wet bedding | ||
| 5 | Forced swimming | Water deprivation | |
| 6 | |||
| 7 | Restraint stress | Cage tilting | |
| 8 | Forced swimming | ||
| 9 | Restraint stress | Bed deprivation | |
| 10 | Wet bedding | ||
| 11 | Restraint stress | Food deprivation | |
| 12 | Forced swimming | Cage tilting | |
| 13 | |||
| 14 | Forced swimming | Cage tilting | |
| 15 | Restraint stress | Wet bedding | |
| 16 | Forced swimming | Night lighting | |
| 17 | Restraint stress | Cage tilting | |
| 18 | Forced swimming | Bed deprivation | |
| 19 | Restraint stress | Night lighting | |
| 20 | |||
| 21 | Cage tilting | ||
| 22 | Lighting | ||
| 23 | Modified Y-maze test * | Bed deprivation | |
| 24 | Open-field test * | Wet bedding | |
| 25 | Y-maze test * | Night lighting | |
| 26 | Restraint stress | Cage tilting | |
| 27 | Novel object recognition test * | ||
| 28 | Novel object recognition test * | Night lighting | |
| 29 | Tail suspension test * | Bed deprivation | |
| 30 | Tail suspension test * | Cage tilting | |
| 31 | Wet bedding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwai, T.; Mishima, R.; Hirayama, S.; Nakajima, H.; Oyama, M.; Watanabe, S.; Fujii, H.; Tanabe, M. SYK-623, a δ Opioid Receptor Inverse Agonist, Mitigates Chronic Stress-Induced Behavioral Abnormalities and Disrupted Neurogenesis. J. Clin. Med. 2024, 13, 608. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13020608
Iwai T, Mishima R, Hirayama S, Nakajima H, Oyama M, Watanabe S, Fujii H, Tanabe M. SYK-623, a δ Opioid Receptor Inverse Agonist, Mitigates Chronic Stress-Induced Behavioral Abnormalities and Disrupted Neurogenesis. Journal of Clinical Medicine. 2024; 13(2):608. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13020608
Chicago/Turabian StyleIwai, Takashi, Rei Mishima, Shigeto Hirayama, Honoka Nakajima, Misa Oyama, Shun Watanabe, Hideaki Fujii, and Mitsuo Tanabe. 2024. "SYK-623, a δ Opioid Receptor Inverse Agonist, Mitigates Chronic Stress-Induced Behavioral Abnormalities and Disrupted Neurogenesis" Journal of Clinical Medicine 13, no. 2: 608. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13020608