Retinal Vascular Changes in Heart Failure with Preserved Ejection Fraction Using Optical Coherence Tomography Angiography
Abstract
:1. Introduction
2. Methods
2.1. Design and Participants
2.2. OCT-A Measurements
2.3. Echocardiographic Analysis
2.4. Statistical Analysis
3. Results
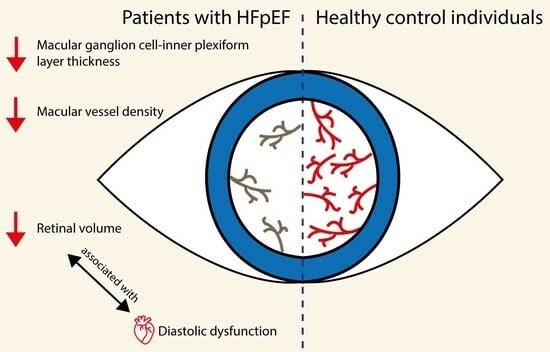
3.1. Retinal Differences in HFpEF
3.2. Associations between OCT-A Markers
3.3. Asymmetry and Impact of Ocular Pathologies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 42201. [Google Scholar] [CrossRef]
- Barandiaran Aizpurua, A.; Sanders-van Wijk, S.; Brunner-La Rocca, H.P.; Henkens, M.; Weerts, J.; Spanjers, M.H.A.; Knackstedt, C.; van Empel, V.P.M. Iron deficiency impacts prognosis but less exercise capacity in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 1304–1313. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Wintergerst, M.W.M.; Falahat, P.; Holz, F.G.; Schaefer, C.; Finger, R.P.; Schahab, N. Retinal and choriocapillaris perfusion are associated with ankle-brachial-pressure-index and Fontaine stage in peripheral arterial disease. Sci. Rep. 2021, 11, 11458. [Google Scholar] [CrossRef]
- Rabiolo, A.; Gelormini, F.; Sacconi, R.; Cicinelli, M.V.; Triolo, G.; Bettin, P.; Nouri-Mahdavi, K.; Bandello, F.; Querques, G. Comparison of methods to quantify macular and peripapillary vessel density in optical coherence tomography angiography. PLoS ONE 2018, 13, e0205773. [Google Scholar] [CrossRef]
- Tewarie, P.; Balk, L.; Costello, F.; Green, A.; Martin, R.; Schippling, S.; Petzold, A. The OSCAR-IB Consensus Criteria for Retinal OCT Quality Assessment. PLoS ONE 2012, 7, e34823. [Google Scholar] [CrossRef] [PubMed]
- Weerts, J.; Barandiarán Aizpurua, A.; Henkens, M.T.H.M.; Lyon, A.; van Mourik, M.J.W.; van Gemert, M.R.A.A.; Raafs, A.; Sanders-van Wijk, S.; Bayés-Genís, A.; Heymans, S.R.B.; et al. The prognostic impact of mechanical atrial dysfunction and atrial fibrillation in heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Nagele, M.P.; Barthelmes, J.; Ludovici, V.; Cantatore, S.; von Eckardstein, A.; Enseleit, F.; Luscher, T.F.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Retinal microvascular dysfunction in heart failure. Eur. Heart J. 2018, 39, 47–56. [Google Scholar] [CrossRef]
- Weerts, J.; Mourmans, S.G.J.; Barandiaran Aizpurua, A.; Schroen, B.L.M.; Knackstedt, C.; Eringa, E.; Houben, A.; van Empel, V.P.M. The Role of Systemic Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction. Biomolecules 2022, 12, 278. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Liu, H.X.; Gao, J. Peripapillary Region Perfusion and Retinal Nerve Fiber Layer Thickness Abnormalities in Diabetic Retinopathy Assessed by OCT Angiography. Transl. Vis. Sci. Technol. 2019, 8, 14. [Google Scholar] [CrossRef]
- Richter, G.M.; Sylvester, B.; Chu, Z.; Burkemper, B.; Madi, I.; Chang, R.; Reznik, A.; Varma, R.; Wang, R.K. Peripapillary microvasculature in the retinal nerve fiber layer in glaucoma by optical coherence tomography angiography: Focal structural and functional correlations and diagnostic performance. Clin. Ophthalmol. 2018, 12, 2285–2296. [Google Scholar] [CrossRef]
- Garcia-Martin, E.; Ruiz-de Gopegui, E.; León-Latre, M.; Otin, S.; Altemir, I.; Polo, V.; Larrosa, J.M.; Cipres, M.; Casasnovas, J.A.; Pablo, L.E. Influence of cardiovascular condition on retinal and retinal nerve fiber layer measurements. PLoS ONE 2017, 12, e0189929. [Google Scholar] [CrossRef]
- Meira-Freitas, D.; Melo, L.A., Jr.; Almeida-Freitas, D.B.; Paranhos, A., Jr. Glaucomatous optic nerve head alterations in patients with chronic heart failure. Clin. Ophthalmol. 2012, 6, 623–629. [Google Scholar] [CrossRef]
- Dehghani, C.; Srinivasan, S.; Edwards, K.; Pritchard, N.; Russell, A.W.; Malik, R.A.; Efron, N. Presence of Peripheral Neuropathy Is Associated with Progressive Thinning of Retinal Nerve Fiber Layer in Type 1 Diabetes. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO234–BIO239. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Shin, H.-W.; Kim, H.; Son, J.; Yoon, H.-J.; Park, H.-S.; Cho, Y.-K.; Han, C.-D.; Nam, C.-W.; Hur, S.-H.; Kim, Y.-N.; et al. Tissue Doppler Imaging as a Prognostic Marker for Cardiovascular Events in Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Soc. Echocardiogr. 2010, 23, 755–761. [Google Scholar] [CrossRef]
- Ohte, N.; Kikuchi, S.; Iwahashi, N.; Kinugasa, Y.; Dohi, K.; Takase, H.; Masai, K.; Inoue, K.; Okumura, T.; Hachiya, K.; et al. Unfavourable outcomes in patients with heart failure with higher preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2022, 24, 293–300. [Google Scholar] [CrossRef]
- Kosmala, W.; Rojek, A.; Przewlocka-Kosmala, M.; Wright, L.; Mysiak, A.; Marwick, T.H. Effect of Aldosterone Antagonism on Exercise Tolerance in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2016, 68, 1823–1834. [Google Scholar] [CrossRef]
- Tromp, J.; Lim, S.L.; Tay, W.T.; Teng, T.K.; Chandramouli, C.; Ouwerkerk, W.; Wander, G.S.; Sawhney, J.P.S.; Yap, J.; MacDonald, M.R.; et al. Microvascular Disease in Patients with Diabetes with Heart Failure and Reduced Ejection Versus Preserved Ejection Fraction. Diabetes Care 2019, 42, 1792–1799. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Qian, Y.; Tang, X.; Ling, H.; Chen, K.; Li, Y.; Gao, P.; Zhu, D. Association of Inter-arm Blood Pressure Difference with Asymptomatic Intracranial and Extracranial Arterial Stenosis in Hypertension Patients. Sci. Rep. 2016, 6, 29894. [Google Scholar] [CrossRef]
- Azad, R.; Sinha, S.; Nishant, P. Asymmetric diabetic retinopathy. Indian J. Ophthalmol. 2021, 69, 3026–3034. [Google Scholar] [CrossRef]
- Liu, G.; Keyal, K.; Wang, F. Interocular Symmetry of Vascular Density and Association with Central Macular Thickness of Healthy Adults by Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 16297. [Google Scholar] [CrossRef]
- Jo, Y.H.; Sung, K.R.; Shin, J.W. Effects of Age on Peripapillary and Macular Vessel Density Determined Using Optical Coherence Tomography Angiography in Healthy Eyes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3492–3498. [Google Scholar] [CrossRef]
- Nieves-Moreno, M.; Martínez-de-la-Casa, J.M.; Morales-Fernández, L.; Sánchez-Jean, R.; Sáenz-Francés, F.; García-Feijoó, J. Impacts of age and sex on retinal layer thicknesses measured by spectral domain optical coherence tomography with Spectralis. PLoS ONE 2018, 13, e0194169. [Google Scholar] [CrossRef]
- Song, W.K.; Lee, S.C.; Lee, E.S.; Kim, C.Y.; Kim, S.S. Macular thickness variations with sex, age, and axial length in healthy subjects: A spectral domain-optical coherence tomography study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3913–3918. [Google Scholar] [CrossRef]


| HFpEF n = 22 | Controls n = 24 | p-Value | |
|---|---|---|---|
| Age, years | 74 [68–80] | 68 [58–77] | 0.027 |
| Female sex, n (%) | 16 (73) | 10 (42) | 0.034 |
| Cardiovascular comorbidities, n (%) | |||
| Atrial fibrillation | 12 (55) | 2 (8) | <0.001 |
| Diabetes mellitus type 2 | 6 (27) | 0 (0) | 0.008 |
| Hypertension | 18 (82) | 12 (50) | 0.024 |
| Dyslipidaemia | 14 (64) | 3 (13) | <0.001 |
| Stroke | 5 (23) | 0 (0) | 0.019 |
| Clinical presentation | |||
| Diastolic blood pressure, mmHg | 81 [66–89] | - | |
| Systolic blood pressure, mmHg | 141 [124–151] | - | |
| NT-proBNP, pg/mL | 553 [302–1277] | - | |
| BMI, kg/m2 | 29 [25–37] | - | |
| Echocardiography | |||
| LVEF, % | 62 [58–66] | - | |
| LVMI, g/m2 | 72 [60–86] | - | |
| e’ septal, cm/s | 6.0 [5.3–7.8] | - | |
| e’ lateral, cm/s | 7.9 [6.2–10.2] | - | |
| E/e’ septal | 14.1 [11.6–19.5] | - | |
| E/e’ lateral | 12.5 [9.3–15.9] | - | |
| E/e’ average | 13.6 [10.7–17.8] | - | |
| TR speed, cm/s | 2.6 [2.3–3.0] | - | |
| RVSP, mmHg | 32.5 [25.0–41.3] | - | |
| LAVI, ml/m2 | 48 [39–55] | - | |
| OCT angiographic measurements | |||
| Central retinal perfusion density | 0.22 [0.19–0.32] | 0.26 [0.21–0.31] | 0.525 |
| Central retinal vessel density, mm−1 | 10.8 [9.2–15.7] | 12.9 [10.5–15.4] | 0.540 |
| Macular vessel density, mm−1 | 19.9 [18.6–20.7] | 21.1 [20.6–21.4] | <0.001 |
| Macular GCIPL thickness, µm | 58.8 [55.8–61.8] | 62.0 [58.6–67.4] | 0.025 |
| Average central retinal thickness, µm | 281 [257–296] | 279 [263–286] | 0.668 |
| Total retinal volume, mm3 | 9.5 [10.1–10.4] | 10.3 [9.9–10.7] | 0.050 |
| FAZ perimeter, mm | 1.92 [1.48–2.18] | 1.98 [1.67–2.39] | 0.632 |
| FAZ area, mm2 | 0.23 [0.13–0.29] | 0.23 [0.16–0.28] | 0.743 |
| FAZ circularity index | 0.76 [0.71–0.80] | 0.71 [0.70–0.78] | 0.279 |
| Β | 95%CI | SE β | Standardized β | p-Value | R2 | |
|---|---|---|---|---|---|---|
| E/e’ septal | 0.09 | 0.03–0.16 | 0.03 | 0.60 | 0.011 | 0.36 |
| Adjusted for age | 0.09 | 0.02–0.16 | 0.03 | 0.59 | 0.015 | 0.37 |
| Adjusted for sex | 0.11 | 0.05–0.17 | 0.03 | 0.70 | 0.002 | 0.53 |
| Adjusted for DM | 0.10 | 0.04–0.17 | 0.03 | 0.66 | 0.013 | 0.47 |
| Adjusted for AF | 0.10 | 0.02–0.18 | 0.04 | 0.67 | 0.017 | 0.37 |
| e’ septal (log(cm/s)) | −3.42 | −5.74–−1.10 | 1.09 | −0.62 | 0.006 | 0.38 |
| Adjusted for age | −3.41 | −5.81–−1.00 | 1.13 | −0.61 | 0.009 | 0.38 |
| Adjusted for sex | −3.73 | −6.07–−1.40 | 1.10 | −0.67 | 0.004 | 0.44 |
| Adjusted for DM | −3.32 | −5.68–−0.96 | 1.11 | −0.60 | 0.009 | 0.41 |
| Adjusted for AF | −3.78 | −6.62–−0.95 | 1.33 | −0.68 | 0.012 | 0.39 |
| E/e’ average | 0.09 | 0.004–0.17 | 0.04 | 0.50 | 0.041 | 0.25 |
| Adjusted for age | 0.09 | 0.006–0.18 | 0.04 | 0.52 | 0.038 | 0.29 |
| Adjusted for sex | 0.12 | 0.04–0.20 | 0.04 | 0.66 | 0.008 | 0.45 |
| Adjusted for DM | 0.10 | 0.01–0.18 | 0.04 | 0.54 | 0.030 | 0.33 |
| Adjusted for AF | 0.09 | −0.01–0.20 | 0.05 | 0.52 | 0.067 | 0.25 |
| OCT Angiographic Measurements | HFpEF n = 22 | Controls n = 24 | p-Value |
|---|---|---|---|
| Central retinal perfusion density | 0.22 [0.19–0.32] | 0.26 [0.21–0.31] | 0.525 |
| OS (n = 21/15) | 0.25 [0.18–0.33] | 0.26 [0.18–0.31] | 0.680 |
| OD (n = 18/18) | 0.24 [0.20–0.31] | 0.25 [0.21–0.31] | 0.606 |
| Central retinal vessel density, mm−1 | 10.8 [9.2–15.7] | 12.9 [10.5–15.4] | 0.540 |
| OS (n = 21/15) | 11.8 [9.3–16.0] | 13.0 [8.9–15.4] | 0.849 |
| OD (n = 18/18) | 11.8 [8.5–15.3] | 12.4 [10.4–14.9] | 0.650 |
| Macular vessel density, mm−1 | 19.9 [18.6–20.7] | 21.1 [20.6–21.4] | <0.001 |
| OS (n = 21/15) | 20.3 [18.8–20.8] | 20.8 [20.5–21.5] | 0.009 |
| OD (n = 18/18) | 20.0 [17.8–20.9] | 21.0 [20.8–21.3] | 0.002 |
| Macular GCIPL thickness, µm | 58.8 [55.8–61.8] | 62.0 [58.6–67.4] | 0.025 |
| OS (n = 20/15) | 60.7 [56.0–63.8] | 61.1 [58.6–64.9] | 0.191 |
| OD (n = 19/18) | 58.2 [53.7–60.1] | 62.2 [59.8–67.4] | 0.004 |
| Average central retinal thickness, µm | 281 [257–296] | 279 [263–286] | 0.668 |
| OS (n = 19/15) | 284 [265–299] | 270 [250–280] | 0.111 |
| OD (n = 20/19) | 287 [256–302] | 282 [261–286] | 0.647 |
| Total retinal volume, mm3 | 9.5 [10.1–10.4] | 10.3 [9.9–10.7] | 0.050 |
| OS (n = 19/15) | 10.2 [9.8–10.4] | 10.3 [10.0–10.6] | 0.354 |
| OD (n = 20/19) | 10.0 [9.5–10.4] | 10.5 [9.9–10.9] | 0.038 |
| FAZ perimeter, mm | 1.92 [1.48–2.18] | 1.98 [1.67–2.39] | 0.632 |
| OS (n = 17/15) | 1.94 [1.52–2.38] | 2.05 [1.85–2.48] | 0.295 |
| OD (n = 14/17) | 1.74 [1.23–2.10] | 1.99 [1.66–2.35] | 0.215 |
| FAZ area, mm2 | 0.23 [0.13–0.29] | 0.23 [0.16–0.28] | 0.743 |
| OS (n = 17/15) | 0.23 [0.14–0.30] | 0.24 [0.21–0.38] | 0.551 |
| OD (n = 14/17) | 0.17 [0.09–0.27] | 0.23 [0.16–0.30] | 0.316 |
| FAZ circularity index | 0.76 [0.71–0.80] | 0.71 [0.70–0.78] | 0.279 |
| OS (n = 17/15) | 0.77 [0.71–0.81] | 0.70 [0.66–0.78] | 0.216 |
| OD (n = 14/17) | 0.75 [0.73–0.78] | 0.72 [0.70–0.77] | 0.262 |
| Left Eye | Right Eye | |||||||
|---|---|---|---|---|---|---|---|---|
| β | St. β | p-Value | R2 | β | St. β | p-Value | R2 | |
| E/e’ septal | 0.02 | 0.112 | 0.680 | 0.013 | 0.17 | 0.687 | 0.003 | 0.471 |
| Adjusted for age | 0.02 | −0.190 | 0.496 | 0.049 | 0.17 | 0.685 | 0.005 | 0.474 |
| Adjusted for sex | 0.05 | 0.249 | 0.349 | 0.220 | 0.17 | 0.720 | 0.004 | 0.491 |
| Adjusted for diabetes mellitus | 0.04 | 0.185 | 0.507 | 0.101 | 0.17 | 0.706 | 0.004 | 0.488 |
| Adjusted for atrial fibrillation | −0.01 | −0.055 | 0.872 | 0.063 | 0.19 | 0.804 | 0.003 | 0.518 |
| e’ septal (log(cm/s)) | −1.12 | −0.168 | 0.519 | 0.028 | −5.77 | −0.677 | 0.003 | 0.458 |
| Adjusted for age | −0.03 | −0.153 | 0.543 | 0.052 | −5.77 | −0.677 | 0.004 | 0.458 |
| Adjusted for sex | −1.66 | −0.250 | 0.340 | 0.149 | −5.82 | −0.682 | 0.004 | 0.459 |
| Adjusted for diabetes mellitus | −0.96 | −0.144 | 0.580 | 0.098 | −5.80 | −0.680 | 0.004 | 0.459 |
| Adjusted for atrial fibrillation | −0.12 | −0.019 | 0.953 | 0.073 | −6.89 | −0.808 | 0.003 | 0.502 |
| E/e’ average | 0.02 | 0.084 | 0.757 | 0.007 | 0.16 | 0.568 | 0.022 | 0.322 |
| Adjusted for age | 0.02 | 0.105 | 0.706 | 0.049 | 0.16 | 0.583 | 0.023 | 0.340 |
| Adjusted for sex | 0.06 | 0.267 | 0.327 | 0.225 | 0.17 | 0.626 | 0.020 | 0.352 |
| Adjusted for diabetes mellitus | 0.03 | 0.132 | 0.633 | 0.085 | 0.16 | 0.577 | 0.025 | 0.329 |
| Adjusted for atrial fibrillation | −0.02 | −0.071 | 0.828 | 0.065 | 0.18 | 0.638 | 0.026 | 0.341 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerts, J.; Raafs, A.G.; Sandhoefner, B.; van der Heide, F.C.T.; Mourmans, S.G.J.; Wolff, N.; Finger, R.P.; Falahat, P.; Wintergerst, M.W.M.; van Empel, V.P.M.; et al. Retinal Vascular Changes in Heart Failure with Preserved Ejection Fraction Using Optical Coherence Tomography Angiography. J. Clin. Med. 2024, 13, 1892. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13071892
Weerts J, Raafs AG, Sandhoefner B, van der Heide FCT, Mourmans SGJ, Wolff N, Finger RP, Falahat P, Wintergerst MWM, van Empel VPM, et al. Retinal Vascular Changes in Heart Failure with Preserved Ejection Fraction Using Optical Coherence Tomography Angiography. Journal of Clinical Medicine. 2024; 13(7):1892. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13071892
Chicago/Turabian StyleWeerts, Jerremy, Anne G. Raafs, Birgit Sandhoefner, Frank C. T. van der Heide, Sanne G. J. Mourmans, Nicolas Wolff, Robert P. Finger, Peyman Falahat, Maximilian W. M. Wintergerst, Vanessa P. M. van Empel, and et al. 2024. "Retinal Vascular Changes in Heart Failure with Preserved Ejection Fraction Using Optical Coherence Tomography Angiography" Journal of Clinical Medicine 13, no. 7: 1892. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm13071892