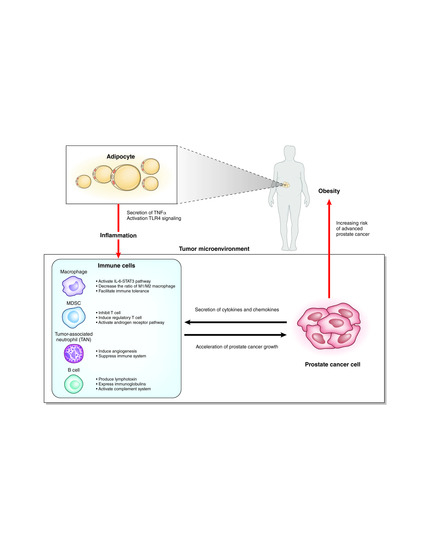
Obesity, Inflammation, and Prostate Cancer
Abstract
:1. Introduction
2. Obesity and Prostate Cancer
3. Obesity and Inflammation
4. Obesity Promotes Prostate Cancer Growth
5. Inflammation in Prostate Cancer
6. Macrophages
7. Myeloid-Derived Suppressor Cells (MDSCs)
8. Neutrophils
9. B Cells and Complements
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arroyo-Johnson, C.; Mincey, K.D. Obesity Epidemiology Worldwide. Gastroenterol. Clin. North Am. 2016, 45, 571–579. [Google Scholar] [CrossRef]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Weitzman, S.A.; Gordon, L.I. Inflammation and cancer: Role of phagocyte-generated oxidants in carcinogenesis. Blood 1990, 76, 655–663. [Google Scholar] [PubMed]
- Cohen, R.J.; Shannon, B.A.; McNeal, J.E.; Shannon, T.; Garrett, K.L. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: A possible link to cancer evolution? J. Urol. 2005, 173, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Ewing, C.M.; Sokoll, L.J.; Elliott, D.J.; Cunningham, M.; De Marzo, A.M.; Isaacs, W.B.; Pavlovich, C.P. Cytokine profiling of prostatic fluid from cancerous prostate glands identifies cytokines associated with extent of tumor and inflammation. Prostate 2008, 68, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Wang, H.; Poutanen, M.; Risbridger, G.P. Increased endogenous estrogen synthesis leads to the sequential induction of prostatic inflammation (prostatitis) and prostatic pre-malignancy. Am. J. Pathol. 2009, 175, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Wilson, B.A.; De Marzo, A.M.; Isaacs, W.B. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 3443–3448. [Google Scholar] [CrossRef]
- DuPre, N.C.; Flavin, R.; Sfanos, K.S.; Unger, R.H.; To, S.; Gazeeva, E.; Fiorentino, M.; De Marzo, A.M.; Rider, J.R.; Mucci, L.A.; et al. Corpora amylacea in prostatectomy tissue and associations with molecular, histological, and lifestyle factors. Prostate 2018, 78, 1172–1180. [Google Scholar] [CrossRef]
- Nakai, Y.; Nonomura, N. Inflammation and prostate carcinogenesis. Int. J. Urol. 2013, 20, 150–160. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef]
- Norrish, A.E.; Ferguson, L.R.; Knize, M.G.; Felton, J.S.; Sharpe, S.J.; Jackson, R.T. Heterocyclic amine content of cooked meat and risk of prostate cancer. J. Natl. Cancer Inst. 1999, 91, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Nelson, W.G.; De Marzo, A.M. The dietary charred meat carcinogen 2-Amino-1-Methyl-6-Phenylimidazo (4,5- b) Pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007, 67, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Torner, J.C.; Lynch, C.F.; Rubenstein, L.M.; Lemke, J.H.; Cohen, M.B.; Lubaroff, D.M.; Wallace, R.B. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control. 1997, 8, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Putnam, S.D.; Cerhan, J.R.; Parker, A.S.; Bianchi, G.D.; Wallace, R.B.; Cantor, K.P.; Lynch, C.F. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann. Epidemiol. 2000, 10, 361–369. [Google Scholar] [CrossRef]
- Discacciati, A.; Orsini, N.; Andersson, S.-O.; Andrén, O.; Johansson, J.-E.; Wolk, A. Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: A population-based prospective study. Br. J. Cancer 2011, 105, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Möller, E.; Wilson, K.M.; Batista, J.L.; Mucci, L.A.; Bälter, K.; Giovannucci, E. Body size across the life course and prostate cancer in the Health Professionals Follow-up Study. Int. J. Cancer 2016, 138, 853–865. [Google Scholar] [CrossRef]
- Juul, A.; Bang, P.; Hertel, N.T.; Main, K.; Dalgaard, P.; Jørgensen, K.; Müller, J.; Hall, K.; Skakkebaek, N.E. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: Relation to age, sex, stage of puberty, testicular size, and body mass index. J. Clin. Endocrinol. Metab. 1994, 78, 744–752. [Google Scholar]
- Discacciati, A.; Orsini, N.; Wolk, A. Body mass index and incidence of localized and advanced prostate cancer-a dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 1665–1671. [Google Scholar] [CrossRef]
- Lima, N.; Cavaliere, H.; Knobel, M.; Halpern, A.; Medeiros-Neto, G. Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Leitzmann, M.F.; Rifai, N.; Kantoff, P.W.; Chen, Y.-C.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Ujike, T.; Uemura, M.; Kawashima, A.; Nagahara, A.; Fujita, K.; Miyagawa, Y.; Nonomura, N. A novel model to predict positive prostate biopsy based on serum androgen level. Endocr. Relat. Cancer 2018, 25, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Branche, B.L.; Howard, L.E.; Hamilton, R.J.; Aronson, W.J.; Terris, M.K.; Cooperberg, M.R.; Amling, C.L.; Kane, C.J. Obesity, Risk of Biochemical Recurrence, and PSADT after Radical Prostatectomy: Results from the SEARCH Database. BJU Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.C.; Howard, L.E.; Sun, S.X.; Cooperberg, M.R.; Kane, C.J.; Aronson, W.J.; Terris, M.K.; Amling, C.L.; Freedland, S.J. Obesity and prostate cancer-specific mortality after radical prostatectomy: Results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Prostate Cancer Prostatic Dis. 2017, 20, 72–78. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Huang, M.; Narita, S.; Numakura, K.; Tsuruta, H.; Saito, M.; Inoue, T.; Horikawa, Y.; Tsuchiya, N.; Habuchi, T. A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate 2012, 72, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Narita, S.; Inoue, T.; Tsuchiya, N.; Satoh, S.; Nanjo, H.; Sasaki, T.; Habuchi, T. Diet-induced macrophage inhibitory cytokine 1 promotes prostate cancer progression. Endocr. Relat. Cancer 2014, 21, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Nara, T.; Narita, S.; Mingguo, H.; Yoshioka, T.; Koizumi, A.; Numakura, K.; Tsuruta, H.; Maeno, A.; Saito, M.; Inoue, T.; et al. Altered miRNA expression in high-fat diet-induced prostate cancer progression. Carcinogenesis 2016, 37, 1129–1137. [Google Scholar] [CrossRef]
- Hu, M.; Xu, H.; Zhu, W.; Bai, P.; Hu, J.; Yang, T.; Jiang, H.; Ding, Q. High-fat diet-induced adipokine and cytokine alterations promote the progression of prostate cancer in vivo and in vitro. Oncol. Lett. 2017, 15, 1607–1615. [Google Scholar] [CrossRef]
- Zhang, T.; Tseng, C.; Zhang, Y.; Sirin, O.; Corn, P.G.; Li-Ning-Tapia, E.M.; Troncoso, P.; Davis, J.; Pettaway, C.; Ward, J.; et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun. 2016, 7, 11674. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Bonavita, E.; Barajon, I.; Garlanda, C.; Mantovani, A.; Jaillon, S. Tumor associated macrophages and neutrophils in cancer. Immunobiology 2013, 218, 1402–1410. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin. Cancer Res. 2018, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.H.; Corrêa, R.; Farinasso, C.M.; de Sant’Ana Dourado, L.P.; Magalhães, K.G. Adipocytes and macrophages interplay in the orchestration of tumor microenvironment: New implications in cancer progression. Front. Immunol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mclaughlin, T.; Shen, L.; Engleman, E.; Mclaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Arendt, L.M.; McCready, J.; Keller, P.J.; Baker, D.D.; Naber, S.P.; Seewaldt, V.; Kuperwasser, C. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013, 73, 6080–6093. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Day, L.L.; Harwood, J.; Ying, C.; St. John, L.N.; Giles, R.; Neeley, C.K.; Pienta, K.J. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia 2006, 8, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Ewing, C.M.; Getzenberg, R.H.; Parsons, J.K.; Isaacs, W.B.; Pavlovich, C.P. Monocyte chemotactic protein-1 (MCP-1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate 2010, 70, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zeigler-Johnson, C.; Morales, K.H.; Lal, P.; Feldman, M. The relationship between obesity, prostate tumor infiltrating lymphocytes and macrophages, and biochemical failure. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Millrud, C.R.; Bergenfelz, C.; Leandersson, K. On the origin of myeloid derived suppressor cells. Oncotarget 2017, 8, 3649–3665. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.-H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef]
- Wang, G.; Lu, X.; Dey, P.; Deng, P.; Wu, C.C.; Jiang, S.; Fang, Z.; Zhao, K.; Konaparthi, R.; Hua, S.; et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016, 6, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Idorn, M.; Køllgaard, T.; Kongsted, P.; Sengeløv, L.; thor Straten, P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol. Immunother. 2014, 63, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Hossain, D.M.S.; Pal, S.K.; Moreira, D.; Duttagupta, P.; Zhang, Q.; Won, H.; Jones, J.; D’Apuzzo, M.; Forman, S.; Kortylewski, M. TLR9-Targeted STAT3 silencing abrogates immunosuppressive activity of myeloid-derived suppressor cells from prostate cancer patients. Clin. Cancer Res. 2015, 21, 3771–3782. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.B.; Singhal, S.; Albelda, S.M. Mouse versus human neutrophils in cancer: A major knowledge gap. Trends Cancer Res. 2017, 3, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Cancer related circulating and tumor-associated neutrophils-subtypes, sources and function. FEBS J. 2018, 285, 4316–4342. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Olson, O.C.; Bhardwaj, P.; Walsh, L.A.; Akkari, L.; Quick, M.L.; Chen, I.-C.; Wendel, N.; Ben-Chetrit, N.; Walker, J.; et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat. Cell. Biol. 2017, 19, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.E.; Yu, Y.-R.A.; He, S.; Wardell, S.E.; Chang, C.-Y.; Kwon, S.; Pillai, R.V.; McDowell, H.B.; Thompson, J.W.; Dubois, L.G.; et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017, 8, 864. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef]
- Patnaik, A.; Swanson, K.D.; Csizmadia, E.; Solanki, A.; Landon-Brace, N.; Gehring, M.P.; Helenius, K.; Olson, B.M.; Pyzer, A.R.; Wang, L.C.; et al. cabozantinib eradicates advanced murine prostate cancer by activating antitumor innate immunity. Cancer Discov. 2017, 7, 750–765. [Google Scholar] [CrossRef]
- Özsoy, M.; Moschini, M.; Fajkovic, H.; Soria, F.; Seitz, C.; Klatte, T.; Gust, K.; Briganti, A.; Karakiewicz, P.I.; Roupret, M.; et al. Elevated preoperative neutrophil–lymphocyte ratio predicts upgrading at radical prostatectomy. Prostate Cancer Prostatic Dis. 2018, 21, 100–105. [Google Scholar] [CrossRef]
- Jang, W.S.; Cho, K.S.; Kim, M.S.; Yoon, C.Y.; Kang, D.H.; Kang, Y.J.; Jeong, W.S.; Ham, W.S.; Choi, Y.D. The prognostic significance of postoperative neutrophil-to-lymphocyte ratio after radical prostatectomy for localized prostate cancer. Oncotarget 2017, 8, 11778–11787. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, R.; Chi, C.; Cai, W.; Zhang, Y.; Qian, H.; Shao, X.; Wang, Y.; Xu, F.; Pan, J.; et al. Systemic immune-inflammation index predicts the combined clinical outcome after sequential therapy with abiraterone and docetaxel for metastatic castration-resistant prostate cancer patients. Prostate 2018, 78, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Boegemann, M.; Schlack, K.; Thomes, S.; Steinestel, J.; Rahbar, K.; Semjonow, A.; Schrader, A.; Aringer, M.; Krabbe, L.-M. The role of the neutrophil to lymphocyte ratio for survival outcomes in patients with metastatic castration-resistant prostate cancer treated with abiraterone. Int. J. Mol. Sci. 2017, 18, 380. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Imamura, R.; Tanigawa, G.; Nakagawa, M.; Hayashi, T.; Kishimoto, N.; Hosomi, M.; Yamaguchi, S. Low serum neutrophil count predicts a positive prostate biopsy. Prostate Cancer Prostatic Dis. 2012, 15, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Hosomi, M.; Tanigawa, G.; Okumi, M.; Fushimi, H.; Yamaguchi, S. Prostatic inflammation detected in initial biopsy specimens and urinary pyuria are predictors of negative repeat prostate biopsy. J. Urol. 2011, 185, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Haas, K.M.; Beck, M.A.; Teague, H. The effects of diet-induced obesity on B cell function. Clin. Exp. Immunol. 2015, 179, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.J.; Somasundaram, R.; Arnold, K.M.; Sims-Mourtada, J. The multifaceted roles of b cells in solid tumors: Emerging treatment opportunities. Target. Oncol. 2017, 12, 139–152. [Google Scholar] [CrossRef]
- Ammirante, M.; Luo, J.-L.; Grivennikov, S.; Nedospasov, S.; Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 2010, 464, 302–305. [Google Scholar] [CrossRef]
- Woo, J.R.; Liss, M.A.; Muldong, M.T.; Palazzi, K.; Strasner, A.; Ammirante, M.; Varki, N.; Shabaik, A.; Howell, S.; Kane, C.J.; et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J. Transl. Med. 2014, 12, 30. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, J. Immunoglobulin G expression in carcinomas and cancer cell lines. FASEB J. 2007, 21, 2931–2938. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, X.; Zhang, L.; Mao, Y.; Zhang, J.; Hao, P.; Li, G.; Lv, P.; Li, Z.; Sun, X.; et al. Human epithelial cancers secrete immunoglobulin g with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003, 63, 6488–6495. [Google Scholar] [PubMed]
- Xu, Y.; Chen, B.; Zheng, S.; Wen, Y.; Xu, A.; Xu, K.; Li, B.; Liu, C. IgG silencing induces apoptosis and suppresses proliferation, migration and invasion in LNCaP prostate cancer cells. Cell. Mol. Biol. Lett. 2016, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Kharghan, V. The role of the complement system in cancer. J. Clin. Investig. 2017, 127, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Macor, P.; Capolla, S.; Tedesco, F. Complement as a biological tool to control tumor growth. Front. Immunol. 2018, 9, 2203. [Google Scholar] [CrossRef] [PubMed]
- Corrales, L.; Ajona, D.; Rafail, S.; Lasarte, J.J.; Riezu-Boj, J.I.; Lambris, J.D.; Rouzaut, A.; Pajares, M.J.; Montuenga, L.M.; Pio, R. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J. Immunol. 2012, 189, 4674–4683. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, W.-J.; Sun, H.-J.; Yang, X.; Wu, Y.-Z. C5b-9 staining correlates with clinical and tumor stage in gastric adenocarcinoma. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Blando, J.; Moore, T.; Hursting, S.; Jiang, G.; Saha, A.; Beltran, L.; Shen, J.; Repass, J.; Strom, S.; DiGiovanni, J. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev. Res. 2011, 4, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shen, M.; Chen, X.; Zhu, R.; Yang, D.-R.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O. Adipocytes affect castration-resistant prostate cancer cells to develop the resistance to cytotoxic action of NK cells with alterations of PD-L1/NKG2D ligand levels in tumor cells. Prostate 2018, 78, 353–364. [Google Scholar] [CrossRef]
- Landim, B.C.; de Jesus, M.M.; Bosque, B.P.; Zanon, R.G.; da Silva, C.V.; Góes, R.M.; Ribeiro, D.L. Stimulating effect of palmitate and insulin on cell migration and proliferation in PNT1A and PC3 prostate cells: Counteracting role of metformin. Prostate 2018, 78, 731–742. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Aguilera Olvera, R.; Lapek, J.D.; Zhang, L.; Wang, W.-B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, J.; Wu, Q.; Chen, J.; Liu, J.; Wang, L.; Chen, C.; Xu, J.; Zhang, H.; Shi, C.; et al. The role of microbiota in the development of colorectal cancer. Int. J. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]



| Gene Symbol | Fold-Change (HFD-fed vs. CD-fed) | p-Value (HFD-fed vs. CD-fed) |
|---|---|---|
| LOC238440 | 17.739 | 0.0163 |
| Ighv6-6 | 12.429 | 0.0170 |
| Ighv3-8 | 10.111 | 0.0048 |
| Ighv14-3 | 9.678 | 0.0395 |
| Igkv10-96 | 8.240 | 0.0248 |
| Mug1 | 5.773 | 0.0068 |
| Snord13 | 5.003 | 0.0002 |
| Igkv10-94 | 4.652 | 0.0260 |
| Adck1 | 4.644 | 0.0261 |
| Itm2a | 4.559 | 0.0223 |
| Igh-VJ558 | 4.509 | 0.0371 |
| Gm830 | 4.480 | 0.0304 |
| Igkv10-95 | 4.070 | 0.0303 |
| Igj | 4.029 | 0.0152 |
| Igh-V3660 | 3.978 | 0.0244 |
| Ighj4 | 3.787 | 0.0016 |
| Igh-VJ558 | 3.765 | 0.0035 |
| Igkv4-55 | 3.733 | 0.0171 |
| Igkv4-59 | 3.716 | 0.0132 |
| Igkv16-104 | 3.596 | 0.0194 |
| Igkv4-91 | 3.542 | 0.0456 |
| Ccl8 | 3.450 | 0.0002 |
| Ighv1-76 | 3.350 | 0.0055 |
| Slc17a4 | 3.333 | 0.0124 |
| LOC637260 | 3.242 | 0.0237 |
| Hc | 3.179 | 0.0337 |
| Tm4sf4 | 3.040 | 0.0424 |
| Ighv1-42 | 3.018 | 0.0333 |
| Igkv5-45 | 3.005 | 0.0095 |
| Ighv14-4 | 3.003 | 0.0255 |
| Ighv1-80 | 2.997 | 0.0095 |
| Igkv4-72 | 2.952 | 0.0091 |
| Ms4a12 | 2.946 | 0.0289 |
| A1cf | 2.911 | 0.0180 |
| Ighv1-77 | 2.876 | 0.0240 |
| Adamts5 | 2.800 | 0.0341 |
| Gm13307 | 2.793 | 0.0064 |
| Clstn2 | 2.784 | 0.0280 |
| Igkj5 | 2.766 | 0.0091 |
| Ighv5-17 | 2.765 | 0.0324 |
| Pdlim3 | 2.763 | 0.0099 |
| Ighj3 | 2.728 | 0.0045 |
| Myh11 | 2.709 | 0.0351 |
| Ighm | 2.708 | 0.0376 |
| Tcrg-V4 | 2.676 | 0.0445 |
| Svep1 | 2.673 | 0.0410 |
| Ighj1 | 2.651 | 0.0290 |
| Iglv1 | 2.632 | 0.0041 |
| Pcp4 | 2.626 | 0.0476 |
| Cpxm2 | 2.617 | 0.0333 |
| Maob | 2.616 | 0.0196 |
| Igkv4-70 | 2.596 | 0.0396 |
| Pgm5 | 2.594 | 0.0453 |
| Cyp2c68 | 2.583 | 0.0168 |
| Igkv4-53 | 2.582 | 0.0451 |
| Ighv1-62-2 | 2.526 | 0.0011 |
| Ppef1 | 2.516 | 0.0118 |
| Acnat1 | 2.511 | 0.0460 |
| Gm13304 | 2.500 | 0.0403 |
| Igkv12-89 | 2.463 | 0.0495 |
| Igh-VX24 | 2.457 | 0.0235 |
| Snord14e | 2.437 | 0.0266 |
| Gm13304 | 2.413 | 0.0414 |
| Thbs4 | 2.352 | 0.0425 |
| Mylk | 2.352 | 0.0185 |
| Cd52 | 2.345 | 0.0066 |
| Abca8a | 2.313 | 0.0066 |
| Kcnab2 | 2.291 | 0.0122 |
| Inmt | 2.290 | 0.0449 |
| Igh-V3660 | 2.272 | 0.0399 |
| Igsf23 | 2.260 | 0.0138 |
| Cd200 | 2.250 | 0.0255 |
| Dkk2 | 2.236 | 0.0418 |
| Acta1 | 2.225 | 0.0401 |
| Hhip | 2.212 | 0.0093 |
| Ecm2 | 2.208 | 0.0109 |
| Lgi2 | 2.180 | 0.0343 |
| Igkv4-62 | 2.167 | 0.0373 |
| Prelp | 2.151 | 0.0243 |
| Igkj1 | 2.143 | 0.0113 |
| Nlrp6 | 2.112 | 0.0417 |
| Gm5485 | 2.062 | 0.0280 |
| Serpini1 | 2.016 | 0.0088 |
| LOC102642448 | 2.014 | 0.0095 |
| Kmo | 2.009 | 0.0386 |
| C4b | 2.009 | 0.0254 |
| Igkv4-53 | 2.001 | 0.0105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8020201
Fujita K, Hayashi T, Matsushita M, Uemura M, Nonomura N. Obesity, Inflammation, and Prostate Cancer. Journal of Clinical Medicine. 2019; 8(2):201. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8020201
Chicago/Turabian StyleFujita, Kazutoshi, Takuji Hayashi, Makoto Matsushita, Motohide Uemura, and Norio Nonomura. 2019. "Obesity, Inflammation, and Prostate Cancer" Journal of Clinical Medicine 8, no. 2: 201. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8020201