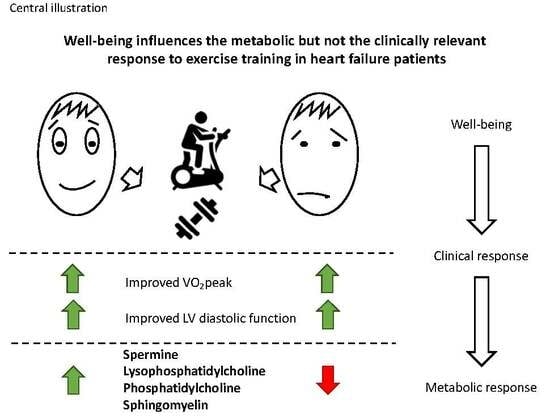
Heterogeneous Metabolic Response to Exercise Training in Heart Failure with Preserved Ejection Fraction
Abstract
:1. Background
2. Methods
2.1. Patient Population
2.2. Metabolomics
2.3. Statistics
3. Results
3.1. Metabolic Response to Exercise Training
3.2. Metabolic Response to Exercise Training Depending on Different Outcome Parameters
3.3. Metabolic Clustering due to Exercise Training in HFpEF Patients
4. Discussion
5. Clinical Relevance
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HFpEF | heart failure with preserved ejection fraction |
| ET | exercise training |
| EX-DHF-P | exercise training in diastolic heart failure-pilot trial |
| UC | usual care group |
| LVMI | left ventricular mass index |
| LAVI | left atrial volume index |
| VO2peak | peak oxygen consumption |
| VE/VCO2 slope | minute ventilation/carbon dioxide production slope |
| E/e’ | ratio of transmitral Doppler early filling velocity to tissue Doppler early diastolic mitral annular velocity |
| FIA | flow injection analysis |
| GEE | generalized estimating equation |
| LDL | low-density lipoprotein |
| BL | baseline |
| FU | follow-up |
| HCA | hierarchical clustering analysis |
References
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Bursi, F.; Weston, S.A.; Redfield, M.M.; Jacobsen, S.J.; Pakhomov, S.; Nkomo, V.T.; Meverden, R.A.; Roger, V.L. Systolic and diastolic heart failure in the community. JAMA 2006, 296, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2014, 11, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Paulus, W.J. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur. Heart J. 2011, 32, 670–679. [Google Scholar] [CrossRef]
- Shah, S.J. Precision Medicine for Heart Failure with Preserved Ejection Fraction: An Overview. J. Cardiovasc. Transl. Res. 2017, 10, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Marwick, T.H. Exercise training for patients with heart failure: A systematic review of factors that improve mortality and morbidity. Am. J. Med. 2004, 116, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Gary, R.A.; Sueta, C.A.; Dougherty, M.; Rosenberg, B.; Cheek, D.; Preisser, J.; Neelon, V.; McMurray, R. Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004, 33, 210–218. [Google Scholar] [CrossRef]
- Edelmann, F.; Gelbrich, G.; Düngen, H.-D.; Fröhling, S.; Wachter, R.; Stahrenberg, R.; Binder, L.; Töpper, A.; Lashki, D.J.; Schwarz, S. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J. Am. Coll. Cardiol. 2011, 58, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.H.; Morgan, T.M.; Stewart, K.P.; Little, W.C. Exercise training in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. Circ. Heart Fail. 2010, 3, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.H.; Herrington, D.M.; Morgan, T.M.; Stewart, K.P.; Hundley, W.G.; Abdelhamed, A.; Haykowsky, M.J. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J. Am. Coll. Cardiol. 2013, 62, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Brubaker, P.H.; Stewart, K.P.; Morgan, T.M.; Eggebeen, J.; Kitzman, D.W. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2012, 60, 120–128. [Google Scholar] [CrossRef]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W., 3rd; Kraus, W.E.; Shah, S.H. Metabolic Dysfunction in Heart Failure: Diagnostic, Prognostic, and Pathophysiologic Insights From Metabolomic Profiling. Curr. Heart Fail. Rep. 2016, 13, 119–131. [Google Scholar] [CrossRef]
- Daskalaki, E.; Easton, C.G.; Watson, D. The Application of Metabolomic Profiling to the Effects of Physical Activity. Curr. Metab. 2014, 2, 233–263. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef]
- Edelmann, F.; Schmidt, A.G.; Gelbrich, G.; Binder, L.; Herrmann-Lingen, C.; Halle, M.; Hasenfuss, G.; Wachter, R.; Pieske, B. Rationale and design of the ’aldosterone receptor blockade in diastolic heart failure’ trial: A double-blind, randomized, placebo-controlled, parallel group study to determine the effects of spironolactone on exercise capacity and diastolic function in patients with symptomatic diastolic heart failure (Aldo-DHF). Eur. J. Heart Fail. 2010, 12, 874–882. [Google Scholar] [CrossRef]
- Perez-Schindler, J.; Kanhere, A.; Edwards, L.; Allwood, J.W.; Dunn, W.B.; Schenk, S.; Philp, A. Exercise and high-fat feeding remodel transcript-metabolite interactive networks in mouse skeletal muscle. Sci. Rep. 2017, 7, 13485. [Google Scholar] [CrossRef]
- Wende, A.R.; Brahma, M.K.; McGinnis, G.R.; Young, M.E. Metabolic Origins of Heart Failure. JACC Basic Transl. Sci. 2017, 2, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Van der Vusse, G.J.; Glatz, J.F.; Stam, H.C.; Reneman, R.S. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol. Rev. 1992, 72, 881–940. [Google Scholar] [CrossRef]
- Nsiah-Sefaa, A.; McKenzie, M. Combined defects in oxidative phosphorylation and fatty acid β-oxidation in mitochondrial disease. Biosci. Rep. 2016, 36, e00313. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Svardal, A.; Oie, E.; Askevold, E.T.; Nymoen, S.H.; Bjorndal, B.; Dahl, C.P.; Gullestad, L.; Berge, R.K.; Aukrust, P. Disturbed carnitine regulation in chronic heart failure–increased plasma levels of palmitoyl-carnitine are associated with poor prognosis. Int. J. Cardiol. 2013, 167, 1892–1899. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Yamaguchi, O.; Takeda, T.; Higuchi, Y.; Hikoso, S.; Taniike, M.; Omiya, S.; Mizote, I.; Matsumura, Y.; Asahi, M.; et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007, 13, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Taneike, M.; Yamaguchi, O.; Nakai, A.; Hikoso, S.; Takeda, T.; Mizote, I.; Oka, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 2010, 6, 600–606. [Google Scholar] [CrossRef]
- Edelmann, F.; Stahrenberg, R.; Polzin, F.; Kockskamper, A.; Dungen, H.D.; Duvinage, A.; Binder, L.; Kunde, J.; Scherer, M.; Gelbrich, G.; et al. Impaired physical quality of life in patients with diastolic dysfunction associates more strongly with neurohumoral activation than with echocardiographic parameters: Quality of life in diastolic dysfunction. Am. Heart J. 2011, 161, 797–804. [Google Scholar] [CrossRef] [PubMed]


| Treatment Groups | ||||
|---|---|---|---|---|
| Variable | All Subjects | Exercise (EX) | Control (CON) | p-Value |
| n = 64 | n = 44 | n = 20 | ||
| Female | 36 (56%) | 24 (55%) | 12 (60%) | 0.79 |
| Age, years | 65 ± 7 | 64 ± 8 | 65 ± 6 | 0.51 |
| BMI, kg/m2 | 31 ± 5 | 31 ± 6 | 31 ± 4 | 0.96 |
| Systolic blood pressure, mmHg | 140 ± 19 | 140 ± 18 | 141 ± 20 | 0.97 |
| Diastolic blood pressure, mmHg | 82 ± 12 | 82 ± 10 | 82 ± 14 | 0.51 |
| Hypertension | 55 (86%) | 38 (86%) | 17 (85%) | 1.00 |
| Obesity | 34 (53%) | 22 (50%) | 12 (60%) | 0.59 |
| Diabetes mellitus | 9 (14%) | 7 (16%) | 2 (10%) | 0.71 |
| Hyperlipidemia | 30 (47%) | 20 (46%) | 10 (50%) | 0.79 |
| Smoking status | 0.65 | |||
| Never smoker | 28 (44%) | 18 (41%) | 10 (50%) | |
| Ex-smoker | 30 (47%) | 21 (48%) | 9 (45%) | |
| Current smoker | 6 (9%) | 5 (11%) | 1 (5%) | |
| NHYA class | 0.15 | |||
| II | 54 (84%) | 35 (80%) | 19 (95%) | |
| III | 10 (16%) | 9 (20%) | 1 (5%) | |
| Echocardiography | ||||
| baseline LVEF, % | 67 ± 7 | 68 ± 7 | 67 ± 7 | 0.59 |
| baseline E/e´ratio | 13.0 ± 3.6 | 12.8 ± 3.2 | 13.5 ± 4.6 | 0.83 |
| baseline LAVI, ml/m2 | 28.0 ± 7.9 | 27.9 ± 7.6 | 28.2 ± 8.8 | 0.88 |
| change in E/e’ ratio | −2.3 (−3.0–1.6) | 0.6 (−0.6–1.8) | <0.001 | |
| change in LAVI, ml/m2 | −3.7 (−4.9–2.4) | 0.3 (−0.7–1.4) | <0.001 | |
| Spiroergometry | ||||
| baseline peak VO2, ml/min/kg | 16.3 ± 4.8 | 16.1 ± 4.9 | 16.7 ± 4.7 | 0.69 |
| baseline VE/VCO2 slope | 27.1 ± 2.9 | 27.5 ± 2.9 | 26.3 ± 2.9 | 0.27 |
| change peak VO2, ml/min/kg | 2.6 (1.8–3.4) | −0.7 (−2.1–0.7) | <0.001 | |
| change VE/VCO2 slope | 0.02 (−5.0 – 5.0) | 0.6 (−4.0 – 4.0) | 0.08 | |
| Medication | ||||
| ACE inhibitor/AT1 receptor antagonist | 42 (66%) | 31 (70%) | 11 (55%) | 0.26 |
| Beta-blocker | 32 (50%) | 20 (45%) | 12 (60%) | 0.42 |
| Diuretics | 29 (45%) | 21 (48%) | 8 (40%) | 0.6 |
| Statins | 17 (27%) | 12 (27%) | 5 (25%) | 0.59 |
| Laboratory parameters | ||||
| LDL-C, mg/dl | 137 ± 32 | 136 ± 31 | 134 ± 34 | 0.79 |
| HDL, mg/dl | 61 ± 21 | 62 ± 23 | 58 ± 16 | 0.698 |
| Hb, g/dl | 14.3 ± 1.2 | 14.5 ± 1.2 | 13.8 ± 1.2 | 0.027 |
| eGFR, ml/min | 85 ± 14 | 85 ± 15 | 86 ± 11 | 0.517 |
| SF-36 (physical) | 43 ± 7 | 43 ± 9 | 44 ± 10 | 0.439 |
| SF-36 (general health) | 58 ± 18 | 56 ± 18 | 59 ± 18 | 0.276 |
| SF-36 (vitality) | 52 ± 20 | 51 ± 21 | 54 ± 17 | 0.149 |
| Exercise | Control | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Class | Metabolite | Baseline | Follow-Up | Baseline | Follow-Up | Training | Control | Interaction |
| Biogenic Amines | Acetylornithine (Ac_Orn) | 0.793 | 0.959 | 0.913 | 0.843 | 0.88 | 0.01 | 0.03 |
| (0.499, 1.615) | (0.558, 1.560) | (0.789, 2.290) | (0.578, 1.388) | |||||
| Acylcarnitines | Carnitine (C0) | 38.32 | 37.71 | 38 | 43.16 | 0.83 | <.01 | 0.05 |
| (32.89, 42.91) | (32.64, 43.79) | (35.02, 43.05) | (38.01, 47.38) | |||||
| Glycerophospholipids | PC aa C28:1 | 3.51 | 3.46 | 3.21 | 3.45 | 0.64 | 0.05 | 0.05 |
| (2.71, 4.37) | (2.75, 4.39) | (2.68, 3.48) | (3.03, 4.15) | |||||
| PC aa C34:2 | 320.56 | 315.63 | 295.54 | 313.66 | 0.35 | 0.01 | 0.05 | |
| (289.39, 359.66) | (278.61, 365.21) | (271.55, 323.82) | (294.72, 341.21) | |||||
| PC aa C36:2 | 197.75 | 193.08 | 177.82 | 194 | 0.61 | 0.02 | 0.03 | |
| (166.88, 219.16) | (155.07, 228.27) | (157.52, 202.85) | (184.85, 212.88) | |||||
| PC ae C44:4 | 0.29 | 0.29 | 0.29 | 0.31 | 0.24 | 0.15 | 0.06 | |
| (0.25, 0.33) | (0.24, 0.32) | (0.27, 0.31) | (0.28, 0.34) | |||||
| sphingolipids | SM C18:0 | 17.59 | 17.22 | 16.4 | 16.64 | 0.04 | 0.32 | 0.04 |
| (14.73, 21.29) | (14.02, 20.03) | (14.02, 18.65) | (15.17, 19.31) | |||||
| SM C24:0 | 12.25 | 11.4 | 11.23 | 11.05 | 0.02 | 0.51 | 0.08 | |
| (10.32, 13.61) | (9.13, 13.51) | (10.22, 12.41) | (9.87, 13.88) | |||||
| SM (OH) C16:1 | 2.66 | 2.64 | 2.4 | 2.69 | 0.08 | 0.27 | 0.07 | |
| (2.09, 3.28) | (2.02, 3.24) | (2.16, 2.75) | (2.01, 2.98) | |||||
| Amino Acid | Glutamine (Gln) | 518.12 | 550.89 | 512.72 | 553.8 | <.01 | 0.89 | 0.09 |
| (465.98, 572.04) | (519.69, 612.37) | (480.11, 611.33) | (448.19, 597.87) | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahls, M.; Friedrich, N.; Pietzner, M.; Wachter, R.; Budde, K.; Hasenfuß, G.; Nauck, M.; Pressler, A.; Felix, S.B.; Edelmann, F.; et al. Heterogeneous Metabolic Response to Exercise Training in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2019, 8, 591. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8050591
Bahls M, Friedrich N, Pietzner M, Wachter R, Budde K, Hasenfuß G, Nauck M, Pressler A, Felix SB, Edelmann F, et al. Heterogeneous Metabolic Response to Exercise Training in Heart Failure with Preserved Ejection Fraction. Journal of Clinical Medicine. 2019; 8(5):591. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8050591
Chicago/Turabian StyleBahls, Martin, Nele Friedrich, Maik Pietzner, Rolf Wachter, Kathrin Budde, Gerd Hasenfuß, Matthias Nauck, Axel Pressler, Stephan B. Felix, Frank Edelmann, and et al. 2019. "Heterogeneous Metabolic Response to Exercise Training in Heart Failure with Preserved Ejection Fraction" Journal of Clinical Medicine 8, no. 5: 591. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm8050591