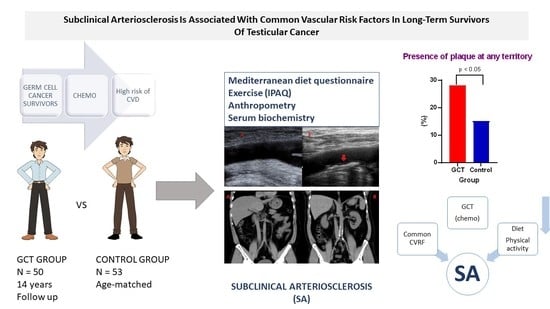
Subclinical Arteriosclerosis is Associated with Common Vascular Risk Factors in Long-Term Survivors of Testicular Cancer
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Subclinical Arteriosclerosis
2.3. Liver Steatosis
2.4. Statistical Analysis
2.5. Ethics
3. Results
Study Participants
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Diemberger, I.; Cosmi, B.; De Ponti, F. ESC position paper on cardiovascular toxicity of cancer treatments: Challenges and expectations—authors’ reply. Intern. Emerg. Med. 2018, 13, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Lahoz, C.; Valdivielso, P.; González-Alegre, M.T.; García-Iglesias, M.F.; Estirado, E.; Mostaza, J.M. Cancer and cardiovascular disease. Clin. Investig. Arterioscler. 2015, 27, 221–225. [Google Scholar] [PubMed]
- Henson, K.E.; Reulen, R.C.; Winter, D.L.; Bright, C.J.; Fidler, M.M.; Frobisher, C.; Guha, J.; Wong, K.F.; Kelly, J.; Edgar, A.B.; et al. Cardiac Mortality among 200 000 Five-Year Survivors of Cancer Diagnosed at 15 to 39 Years of Age: The Teenage and Young Adult Cancer Survivor Study. Circulation 2016, 134, 1519–1531. [Google Scholar] [CrossRef]
- Cheng, L.; Albers, P.; Berney, D.M.; Feldman, D.R.; Daugaard, G.; Gilligan, T.; Looijenga, L.H.J. Testicular cancer. Nat. Rev. Dis. Prim. 2018, 4, 29. [Google Scholar] [CrossRef]
- Huddart, R.A.; Norman, A.; Shahidi, M.; Horwich, A.; Coward, D.; Nicholls, J.; Dearnaley, D.P. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J. Clin. Oncol. 2003, 21, 1513–1523. [Google Scholar] [CrossRef]
- van Den Belt-Dusebout, A.W.; Nuver, J.; de Wit, R.; Gietema, J.A.; Ten Bokkel Huinink, W.W.; Rodrigus, P.T.R.; Schimmel, E.C.; Aleman, B.M.P.; van Leeuwen, F.E. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J. Clin. Oncol. 2006, 24, 467–475. [Google Scholar] [CrossRef]
- Haugnes, H.S.; Wethal, T.; Aass, N.; Dahl, O.; Klepp, O.; Langberg, C.W.; Wilsgaard, T.; Bremnes, R.M.; Fosså, S.D. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: A 20-year follow-up study. J. Clin. Oncol. 2010, 28, 4649–4657. [Google Scholar] [CrossRef]
- Abouassaly, R.; Fossa, S.D.; Giwercman, A.; Kollmannsberger, C.; Motzer, R.J.; Schmoll, H.-J.; Sternberg, C.N. Sequelae of Treatment in Long-term Survivors of Testis Cancer. Eur. Urol. 2011, 60, 516–526. [Google Scholar] [CrossRef]
- Honecker, F.; Aparicio, J.; Berney, D.; Beyer, J.; Bokemeyer, C.; Cathomas, R.; Clarke, N.; Cohn-Cedermark, G.; Daugaard, G.; Dieckmann, K.-P.; et al. ESMO Consensus Conference on testicular germ cell cancer: Diagnosis. treatment and follow-up. Ann. Oncol. 2018, 29, 1658–1686. [Google Scholar] [CrossRef]
- Ness, K.K.; Hudson, M.M.; Ginsberg, J.P.; Nagarajan, R.; Kaste, S.C.; Marina, N.; Whitton, J.; Robison, L.L.; Gurney, J.G. Physical Performance Limitations in the Childhood Cancer Survivor Study Cohort. J. Clin. Oncol. 2009, 27, 2382–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuver, J.; Smit, A.J.; Wolffenbuttel, B.H.R.; Sluiter, W.J.; Hoekstra, H.J.; Sleijfer, D.T.; Gietema, J.A. The Metabolic Syndrome and Disturbances in Hormone Levels in Long-Term Survivors of Disseminated Testicular Cancer. J. Clin. Oncol. 2005, 23, 3718–3725. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Mauriello, A.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Cardillo, C.; Melino, G.; Di Daniele, N. Arterial ageing: From endothelial dysfunction to vascular calcification. J. Intern. Med. 2017, 281, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Wang, T.K.; van Pelt, N.C.; Horne, A.M.; Mason, B.H.; Ames, R.W.; Grey, A.B.; Ruygrok, P.N.; Gamble, G.D.; Reid, I.R. Abdominal aortic calcification on vertebral morphometry images predicts incident myocardial infarction. J. Bone Miner. Res. 2010, 25, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Veglia, F.; de Faire, U.; Giral, P.; Rauramaa, R.; Smit, A.J.; Kurl, S.; Ravani, A.; Frigerio, B.; Sansaro, D.; et al. Carotid plaque-thickness and common carotid IMT show additive value in cardiovascular risk prediction and reclassification. Atherosclerosis 2017, 263, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representati. Atherosclerosis 2016, 252, 207–274. [Google Scholar] [CrossRef] [Green Version]
- Conroy, R.M.; Pyorala, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetiere, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Hear. J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.F.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Martos Dauer, A.; Gual Solé, A.; Llopis Llácer, J.J. The "standard drink unit" as a simplified record of alcoholic drink consumption and its measurement in Spain. Med. Clin. 1999, 112, 446–450. [Google Scholar]
- Adults, E.P. On D.E. and T. of H.B.C. in Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation. and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Friera, L.; Peñalvo, J.L.; Fernández-Ortiz, A.; Ibañez, B.; López-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; Martínez de Vega, V.; et al. Prevalence. Vascular Distribution. and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef] [Green Version]
- Sandfort, V.; Bluemke, D.A. CT calcium scoring. History. current status and outlook. Diagn. Interv. Imaging 2017, 98, 3–10. [Google Scholar] [CrossRef]
- Allison, M.A.; Budoff, M.J.; Nasir, K.; Wong, N.D.; Detrano, R.; Kronmal, R.; Takasu, J.; Criqui, M.H. Ethnic-Specific Risks for Atherosclerotic Calcification of the Thoracic and Abdominal Aorta (from the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2009, 104, 812–817. [Google Scholar] [CrossRef] [Green Version]
- An, C.; Lee, H.-J.; Lee, H.S.; Ahn, S.S.; Choi, B.W.; Kim, M.-J.; Chung, Y.E. CT-based abdominal aortic calcification score as a surrogate marker for predicting the presence of asymptomatic coronary artery disease. Eur. Radiol. 2014, 24, 2491–2498. [Google Scholar] [CrossRef]
- Lee, S.S.; Park, S.H. Radiologic evaluation of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 7392–7402. [Google Scholar] [CrossRef]
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 2018, 10, 530–542. [Google Scholar] [CrossRef]
- Sagstuen, H.; Aass, N.; Fosså, S.D.; Dahl, O.; Klepp, O.; Wist, E.A.; Wilsgaard, T.; Bremnes, R.M. Blood pressure and body mass index in long-term survivors of testicular cancer. J. Clin. Oncol. 2005, 23, 4980–4990. [Google Scholar] [CrossRef] [Green Version]
- Haugnes, H.; Aass, N.; Fossa, S.; Dahl, O.; Klepp, O.; Wist, E.; Svartberg, J.; Wilsgaard, T.; Bremnes, R. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann. Oncol. 2006, 18, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Willemse, P.M.; Burggraaf, J.; Hamdy, N.A.T.; Weijl, N.I.; Vossen, C.Y.; van Wulften, L.; van Steijn-van Tol, A.Q.M.J.; Rosendaal, F.R.; Osanto, S. Prevalence of the metabolic syndrome and cardiovascular disease risk in chemotherapy-treated testicular germ cell tumour survivors. Br. J. Cancer 2013, 109, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kosmalski, M.; Kasznicki, J.; Drzewoski, J. Relationship between ultrasound features of nonalcoholic fatty liver disease and cardiometabolic risk factors in patients with newly diagnosed type 2 diabetes. Polish Arch. Intern. Med. 2013, 123, 436–442. [Google Scholar] [CrossRef]
- Bandak, M.; Jørgensen, N.; Juul, A.; Lauritsen, J.; Oturai, P.S.; Mortensen, J.; Hojman, P.; Helge, J.W.; Daugaard, G. Leydig cell dysfunction. systemic inflammation and metabolic syndrome in long-term testicular cancer survivors. Eur. J. Cancer 2017, 84, 9–17. [Google Scholar] [CrossRef]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef]
- Brea, A.; Puzo, J. Non-alcoholic fatty liver disease and cardiovascular risk. Int. J. Cardiol. 2013, 167, 1109–1117. [Google Scholar] [CrossRef]
- Brea, A.; Mosquera, D.; Martin, E.; Arizti, A.; Cordero, J.L.; Ros, E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: A case-control study. Arter. Thromb. Vasc. Biol. 2005, 25, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Björnson, E.; Packard, C.J.; Adiels, M.; Andersson, L.; Matikainen, N.; Söderlund, S.; Kahri, J.; Sihlbom, C.; Thorsell, A.; Zhou, H.; et al. Investigation of human apoB48 metabolism using a new. integrated non-steady-state model of apoB48- and apoB100 kinetics. J. Intern. Med. 2019, 285, 5. [Google Scholar] [CrossRef] [Green Version]
- Mancera-Romero, J.; Sánchez-Chaparro, M.A.; Rioja, J.; Ariza, M.J.; Olivecrona, G.; González-Santos, P.; Valdivielso, P. Fasting apolipoprotein B48 is a marker for peripheral arterial disease in type 2 diabetes. Acta Diabetol. 2013, 50, 383–389. [Google Scholar] [CrossRef]
- Valdivielso, P.; Puerta, S.; Rioja, J.; Alonso, I.; Ariza, M.J.; Sánchez-Chaparro, M.A.; Palacios, R.; González-Santos, P. Postprandial apolipoprotein B48 is associated with asymptomatic peripheral arterial disease: A study in patients with type 2 diabetes and controls. Clin. Chim. Acta 2010, 411, 433–437. [Google Scholar] [CrossRef]
- Alipour, A.; Valdivielso, P.; Elte, J.W.F.; Janssen, H.W.; Rioja, J.; van der Meulen, N.; van Mechelen, R.; Njo, T.L.; Gonzalez-Santos, P.; Rietveld, A.P.; et al. Exploring the value of apoB48 as a marker for atherosclerosis in clinical practice. Eur. J. Clin. Investig. 2012, 42, 702–708. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.A.; Klop, B.; Eskes, S.A.; van der Loos, T.L.; Klessens-Godfroy, F.J.; Wiebolt, J.; Janssen, H.W.; Westerman, E.M.; Castro Cabezas, M. The postprandial situation as a pro-inflammatory condition. Clin. Investig. Arter. 2014, 26, 184–192. [Google Scholar] [CrossRef] [PubMed]



| Total (103) | Controls (53) | Patients (50) | p value | |
|---|---|---|---|---|
| Age (Years) | 48.5 (7.2) | 49.5 (7.6) | 47.5 (6.7) | |
| Age (Diagnosis) | 33.2 (8.5) | |||
| Years Since Diagnosis | 14.3 (6.8) | |||
| Histology (%) | ||||
| Seminomatous | 24 (48) | |||
| Non-Seminomatous | 26 (52) | |||
| Therapy (%) | ||||
| Carboplatin | 9 (18) | |||
| Etoposide + Platin | 8 (16) | |||
| Bleomicin + Etoposide + Platin | 22 (44) | |||
| Others | 9 (18) | |||
| Unknown | 2 (4) | |||
| Comorbidities (%) | ||||
| Smokers | 18 (17.5) | 8 (15.1) | 10 (20) | |
| Metabolic Syndrome | ||||
| Former smokers | 23 (22.3) | 10 (18.9) | 13 (26) | |
| Drinkers | 8 (7.8) | 5 (9.4) | 3 (6) | |
| High Blood Pressure | 19 (18.4) | 9 (17) | 10 (20) | |
| Diabetes | 5 (4.9) | 2 (3.8) | 3 (6) | |
| Hyperlipidemia | 22 (21.4) | 13 (24.5) | 9 (18) | |
| Metabolic Syndrome | 28 (27) | 15 (27) | 13 (26) | |
| Cardiovascular Risk | ||||
| Score | 1 (0–2) | 1 (0–2) | 1 (0–2) | |
| Score ≥ 5 (%) | 10 (9.7) | 6 (11.32) | 4 (8) | |
| High Cardiovascular Risk (%) * | 12 (11.6) | 6 (11.3) | 6 (12) | |
| Familiar History of coronary disease | 20 (19.4) | 14 (26.4) | 6 (12) | = 0.06 |
| MEDAS | 9.17 (2.5) | 9.45 (2.63) | 8.86 (2.33) | |
| Adherence to Mediterranean Diet (%) | 76 (74) | 43 (81) | 33 (66) | |
| IPAQ (%) | <0.05 | |||
| Sedentary | 15 (14.6) | 4 (7.5) | 11 (22) | |
| Medium Physical Activity | 44 (42.7) | 23 (43.4) | 21 (42) | |
| High Physical Activity | 44 (42.7) | 26 (49.1) | 18 (36) | |
| Body Weight (kg) | 83.5 (11.16) | 81.4 (9.86) | 85.7 (12.11) | <0.05 |
| Body Mass Index (kg/m2) | 27.6 (8.48) | 26.85 (3.17) | 26.91 (3.39) | |
| Waist Circumference (cm) | 100 (12) | 98 (9) | 103 (15) | = 0.06 |
| Systolic Blood Pressure (mm Hg) | 134 (13) | 133 (11) | 135 (15) | |
| Diastolic Blood Pressure (mm Hg) | 86 (9) | 85 (8) | 88 (10) | |
| Total (103) | Controls (53) | Patients (50) | p value | |
|---|---|---|---|---|
| Hb (g/dL) | 15.28 (1.8) | 14.93 (2.17) | 15.66 (1.2) | < 0.05 |
| MCV (fL) | 89.91 (6.94) | 90.73 (6.4) | 89.04 (7.45) | |
| MCH (pg) | 29.85 (2.44) | 29.58 (2.4) | 30.13 (2.46) | |
| RDW (%) | 13.3 (1.03) | 13.6 (0.77) | 12.9 (1.16) | |
| Platelets (10 × 9/L) | 239,669 (62,304) | 227,792 (65,569) | 252,260 (6600) | < 0.05 |
| Total Leukocytes (10 x 9/L) | 6550 (1788 | 6611 (1790) | 6527 (1803) | |
| Neutrophyles (10 x 9/L) | 3722 (1490) | 3668 (1511) | 3781 (1481) | |
| Glucose (mg/dL) | 94 (9) | 93 (7) | 94 (11) | |
| Insulin (µmol/L) | 9.13 (5.88) | 8.19 (4.7) | 10.93 (6.83) | |
| Total Cholesterol (mg/dL) | 195 (33) | 194 (33) | 196 (34) | |
| HDL Cholesterol (mg/dL) | 53 (14) | 58 (14) | 48 (12) | < 0.05 |
| LDL Cholesterol (mg/dL) | 117 (29) | 114 (28) | 121 (29) | |
| Triglycerides (mg/dL) | 123 (73) | 115 (62 | 132 (83) | |
| Uric Acid (mg/dL) | 5.7 (1.52) | 5.79 (1.22) | 5.78 (1.8) | |
| Creatinine (mg/dL) | 0.9 (0.15) | 0.86 (0.11) | 0.94 (0.17) | |
| eGFR (ml/min/1,73 m2) | 87 (6) | 89 (3) | 85 (8) | |
| Urea (mg/dL) | 35 (10) | 34 (8) | 37 (11) | |
| Total Bilirubin (mg/dL) | 0.7 (0.3) | 0.75 (0.33) | 0.64 (0.3) | |
| AST (U/L) | 28 (9) | 27 (7) | 33 (14) | |
| ALT (U/L) | 33 (16) | 30 (15) | 36 (17) | |
| GGT (U/L) | 26 (16–42.25) | 19 (14–30) | 38 (21–57) | <0.05 |
| Iron (µg/dL) | 97 (36) | 94 (35) | 99 (37) | |
| hsCRP (mg/L) | 1.92 (0.71–3.17) | 1.99 (0.61–2.75) | 1.88 (0.91–3.55) | |
| APOB (mg/dL) | 103 (15) | 104 (16) | 103 (14) | |
| APOB48 (mg/dL) | 7.71 (3.79) | 7.50 (2.65) | 7.92 (4.71) | |
| HOMA-IR | 2.14 (1.48) | 1.90 (1.16) | 2.40 (1.73) | = 0.08 |
| Total (103) | Controls (53) | Patients (50) | p value | |
|---|---|---|---|---|
| Ultrasound | ||||
| RCCA | ||||
| IMT (mm) | 0.78 (0.17) | 0.80 (0.19) | 0.76 (0.15) | |
| Plaque (%) | 24 (16) | 10 (19) | 14 (28) | |
| LCCA | ||||
| IMT (mm) | 0.79 (0.17) | 0.80 (0.17) | 0.78 (0.17) | |
| Plaque (%) | 18 (12) | 8 (15) | 10 (20) | |
| RFA | ||||
| IMT (mm) | 0.82 (0.35) | 0.80 (0.36) | 0.84 (0.34) | |
| Plaque (%) | 27 (18) | 9 (17) | 18 (36) | <0.05 |
| LFA | ||||
| IMT (mm) | 0.84 (0.34) | 0.85 (0.35) | 0.83 (0.32) | |
| Plaque (%) | 89 (20) | 15 (28) | 15 (30) | |
| Total Plaques | 32 (21) | 32 (15) | 57 (28) | <0.05 |
| Averaged IMT | 0.81 (0.20) | 0.81 (0.20) | 0.80 (0.19) | |
| Abdominal CT | ||||
| Zone 1 (Ag)1 | 0 (0–0) | 0 (0–0) | 0 (0–1.37) | <0.05 |
| Zone 2 (Ag) | 0 (0–51.2) | 0 (0–32.5) | 0 (0–117.45) | |
| Zone 3 (Ag) | 0 (0–142.3) | 0 (0–71.6) | 0 (0–277.85) | |
| Total (Ag) | 0 (0–296.6) | 0 (0–171) | 0 (0–455.07) | |
| Abnormal AACS (%) | 48 (46.61) | 23 (43.4) | 25 (50) | |
| Liver steatosis1 | 20 (19.4) | 6 (11.3) | 14 (28) | <0.05 |
| Factor | OR (95% Confidence Interval) |
|---|---|
| Age (by Year) | 1.154 (1.053–1.264) |
| HDL Cholesterol (by mmol/L) | 0.929 (0.887–0.973) |
| Apolipoprotein B (by mg/dL) | 1.068 (1.039–1.146) |
| Smoking | 13.290 (2.370–74.512) |
| Hypertension | 16.00 (1.840–138.402) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espíldora-Hernández, J.; Díaz-Antonio, T.; Baena-Espinar, J.; Alonso-Calderón, I.; Rioja, J.; Alba-Conejo, E.; Valdivielso, P.; Sánchez-Chaparro, M.-Á. Subclinical Arteriosclerosis is Associated with Common Vascular Risk Factors in Long-Term Survivors of Testicular Cancer. J. Clin. Med. 2020, 9, 971. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm9040971
Espíldora-Hernández J, Díaz-Antonio T, Baena-Espinar J, Alonso-Calderón I, Rioja J, Alba-Conejo E, Valdivielso P, Sánchez-Chaparro M-Á. Subclinical Arteriosclerosis is Associated with Common Vascular Risk Factors in Long-Term Survivors of Testicular Cancer. Journal of Clinical Medicine. 2020; 9(4):971. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm9040971
Chicago/Turabian StyleEspíldora-Hernández, Javier, Tania Díaz-Antonio, Javier Baena-Espinar, Inmaculada Alonso-Calderón, José Rioja, Emilio Alba-Conejo, Pedro Valdivielso, and Miguel-Ángel Sánchez-Chaparro. 2020. "Subclinical Arteriosclerosis is Associated with Common Vascular Risk Factors in Long-Term Survivors of Testicular Cancer" Journal of Clinical Medicine 9, no. 4: 971. https://0-doi-org.brum.beds.ac.uk/10.3390/jcm9040971