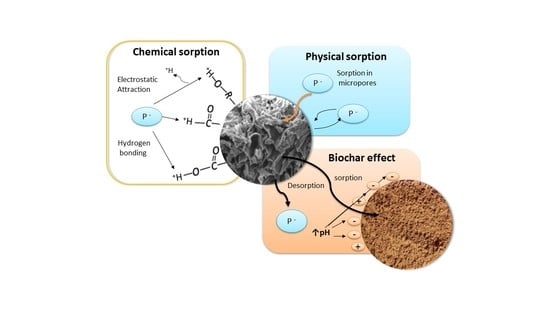
Biochar Chemistry in a Weathered Tropical Soil: Kinetics of Phosphorus Sorption
Abstract
:1. Introduction
2. Material and Methods
2.1. Biochar Formation and Characterization
2.2. Soil Collection and Preparation
2.3. Sorption Studies
2.4. Statistical Methods
3. Results and Discussion
3.1. Biochar and Soil/BC Mixture Characteristics
3.2. Sorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Fao Statistical Yearbook 2014: Latin America and the Caribbean Food and Agriculture; FAO: Santiago, Chile, 2014. [Google Scholar]
- Baligar, V.C.; Fageria, N.K.; Eswaran, H.; Wilson, M.J.; He, Z. Nature and properties of red soils of the world. In The Red Soils of China; Wilson, M.J., He, Z., Yang, X., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 7–27. [Google Scholar]
- Sato, S.; Comerford, N.B. Organic anions and phosphorus desorption and bioavailability in a humid Brazilian ultisol. Soil Sci. 2006, 171, 695–705. [Google Scholar] [CrossRef]
- Gonçalves, J.L.d.M.; Firme, D.J.; Novais, R.F.; Ribeiro, A.C. Cinética de adsorção de fósforo em solos de cerrado. Rev. Bras. Ciência Solo 1985, 9, 107–111. [Google Scholar]
- Smethurst, P.J. Soil solution and other soil analyses as indicators of nutrient supply: A review. For. Ecol. Manag. 2000, 138, 397–411. [Google Scholar] [CrossRef]
- Fox, R.L. External phosphorus requirements of Crops1. In Chemistry in the Soil Environment; Dowdy, R.H., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1981; pp. 223–239. [Google Scholar]
- Mendham, D.S. Predicting Phosphorus Limitations in Eucalyptus Plantations. Ph.D. Thesis, University of Tasmania, Sandy Bay, Tasmania, 1998; p. 234. [Google Scholar]
- Golchin, A.; Clarke, P.; Baldock, J.A.; Higashi, T.; Skjemstad, J.O.; Oades, J.M. The effects of vegetation and burning on the chemical composition of soil organic matter in a volcanic ash soil as shown by C-13 NMR spectroscopy.1. Whole soil and humic acid fraction. Geoderma 1997, 76, 155–174. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macedo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.M.; Comerford, N.; Guerrini, I.A.; Falcao, N.P.S.; Reeves, J.B. Sorption and desorption of phosphate on biochar and biochar-soil mixtures. Soil Use Manag. 2013, 29, 306–314. [Google Scholar] [CrossRef]
- Parvage, M.M.; Ulén, B.; Eriksson, J.; Strock, J.; Kirchmann, H. Phosphorus availability in soils amended with wheat residue char. Biol. Fertil. Soils 2013, 49, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; CaiJi, Z.; Liu, J.; Wang, H.; Ren, T.; Gai, X.; Hi, B.; Liu, H. Short-term effects of maize residue biochar on phosphorus availability in two soils with different phosphorus sorption capacities. Biol. Fertil. Soils 2015, 51, 113–122. [Google Scholar] [CrossRef]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The ‘Terra Preta’ phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rondon, M. Bio-char soil management on highly weathered soils in the humid tropics. Biol. Approaches Sustain. Soil Syst. 2006, 113, 517–530. [Google Scholar]
- Wang, T.; Camps-Arbestain, M.; Hedley, M.; Bishop, P. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Nelson, N.O.; Agudelo, S.C.; Yuan, W.; Gan, J. Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci. 2011, 176, 218–226. [Google Scholar] [CrossRef]
- Cui, H.-J.; Wang, M.K.; Fu, M.-L.; Ci, E. Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phos-phate adsorbed on ferrihydrite using rice straw-derived biochar. J. Soils Sediments 2011, 11, 1135–1141. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 1962, 26, 31–36. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface-chemistry of carbon-blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento (Ed.) Normative Instruction n. 28. Manual de Métodos Analíticos Oficiais para Fertilizantes Min-erais, Orgânicos, Organominerais e Corretivos; 28. Brasília, DF, 31, jul, Diário Oficial da União: Brasília, Brazil, 2007; p. 11. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Raij, B.V.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C.E. Recomendações de Adubação e Calagem Para o Estado de São Paulo, 2nd ed.; Instituto Agronomico: Campinas, Brasil, 1996. [Google Scholar]
- Glaser, B.; Balashov, E.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in density fractions of anthropogenic soils of the Brazilian Amazon region. Org. Geochem. 2000, 31, 669–678. [Google Scholar] [CrossRef]
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and mineral-nutrition properties of sand-based turfgrass root zones amended with biochar. Agron. J. 2010, 102, 1627–1631. [Google Scholar] [CrossRef]
- Pereira, R.G.; Heinemann, A.B.; Madari, B.E.; de Melo Carvalho, M.T.; Kliemann, H.J.; dos Santos, A.P. Transpiration response of upland rice to water deficit changed by different levels of eucalyptus biochar. Pesqui. Agropecu. Bras. 2012, 47, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Tryon, E.H. Effect of charcoal on certain physical, chemical, and biological properties of forest soils. Ecol. Monogr. 1948, 18, 81–115. [Google Scholar] [CrossRef]
- Falcão, N.P.S.; Moreira, A.; Comerford, N.B. A fertilidade dos solos de Terra Preta de Índio da Amazônia Central. In As Terras Pretas de Índio da Amazônia: Sua Caracterização e Uso Deste Conhecimento na Criação de Novas Áreas, 1st ed.; Teixeira, W.G., Kern, D.C., Madari, B.E., Lima, H.N., Woods, W., Eds.; Embrapa Amazônia Ocidental: Manaus, Brazil, 2010; pp. 189–200. [Google Scholar]
- Regazzi, A.J.; Silva, C.H.O. Testes para verificar a igualdade de parâmetros e a identidade de modelos de regressão não-linear em dados de experimento com delineamento em blocos casualizados. Rev. Ceres 2010, 57, 315–320. [Google Scholar] [CrossRef]
- Gray, D.M.; Dighton, J. Mineralization of forest litter nutrients by heat and combustion. Soil Biol. Biochem. 2006, 38, 1469–1477. [Google Scholar] [CrossRef]
- Strauss, R.; Brummer, G.W.; Barrow, N.J. Effects of crystallinity of goethite.1. Preparation and properties of goethites of differing crystallinity. Eur. J. Soil Sci. 1997, 48, 87–99. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Hogue, B.A.; Inglett, P.W. Nutrient release from combustion residues of two contrasting herbaceous vegetation types. Sci. Total Environ. 2012, 431, 9–19. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J. Biochar effects on soil nutrient transformation. In Biochar for Environmental Man-agement: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 251–270. [Google Scholar]
- Raison, R.J.; Khanna, P.K.; Woods, P.V. Mechanisms of element transfer to the atmosphere during vegetation fires. Can. J. For. Res. -Rev. Can. Rech. For. 1985, 15, 132–140. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Schneider, F.; Haderlein, S.B. Potential effects of biochar on the availability of phosphorus—Mechanistic insights. Geoderma 2016, 277, 83–90. [Google Scholar] [CrossRef]
- Weng, L.P.; Van Riemsdijk, W.H.; Hiemstra, T. Humic nanoparticles at the oxide-water interface: Interactions with phosphate ion adsorption. Environ. Sci. Technol. 2008, 42, 8747–8752. [Google Scholar] [CrossRef]
- Mahan, B.M.; Myer, R.J. Química um Curso Universitário; Edgard Blücher: São Paulo, Brazil, 2002; p. 5. [Google Scholar]
- Barros, N.F.; Filho; Comerford, N.B. Phosphorus sorption, desorption and resorption by soils of the Brazilian Cerrado support-ing eucalypt. Biomass Bioenergy 2005, 28, 229–236. [Google Scholar] [CrossRef]
- Hachiya, K.; Sasaki, M.; Saruta, Y.; Mikami, N.; Yasunaga, T. Static and kinetic-studies of adsorption desorption of metal-ions on a gamma-al2o3 surface. 1. Static study of adsorption desorption. J. Phys. Chem. 1984, 88, 23–27. [Google Scholar] [CrossRef]
- Strauss, R.; Brummer, G.W.; Barrow, N.J. Effects of crystallinity of goethite. 2. Rates of sorption and desorption of phosphate. Eur. J. Soil Sci. 1997, 48, 101–114. [Google Scholar] [CrossRef]
- Papelis, C.; Roberts, P.V.; Leckie, J.O. Modeling the rate of cadmium and selenite adsorption on microporous and mesoporous transition aluminas. Environ. Sci. Technol. 1995, 29, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus sorption and availability from biochars and soil/biochar mixtures. Clean-Soil Air Water 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Murphy, P.N.C.; Stevens, R.J. Lime and gypsum as source measures to decrease phosphorus loss from soils to water. Water Air Soil Pollut. 2010, 212, 101–111. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]






| Soil Chemical Properties | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Ca | Mg | K | Al | H+Al | CEC | P | Zn | Fe | Mn | B | Cu | OM |
| CaCl2 | mmolc kg−1 | mg kg−1 | g kg−1 | ||||||||||
| 4.1 | 3 | 1 | 1 | 11 | 67 | 71 | 6 | 0.1 | 58 | 1 | 0.1 | 1 | 16 |
| Soil/BC Mixture Chemical Properties | |||||||||||||
| 8.0 | 70 | 13 | 41 | 0 | 7 | 132 | 60 | 0.8 | 16 | 7 | 5 | 0.4 | 27 |
| Fast Biochar Chemical Properties | |||||||||||||
| N | P | K | OM | C | C/N | Ca | Mg | S | Na | Cu | Fe | Mn | Zn |
| g kg−1(dry weight) | mg kg–1 (dry weight) | ||||||||||||
| 06 | 1.48 | 16 | 550 | 310 | 48 | 21 | 2 | 190 | 580 | 22 | 4500 | 264 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, M.M.; Comerford, N.B.; Behling, M.; de Abreu, D.C.; Guerrini, I.A. Biochar Chemistry in a Weathered Tropical Soil: Kinetics of Phosphorus Sorption. Agriculture 2021, 11, 295. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11040295
Morales MM, Comerford NB, Behling M, de Abreu DC, Guerrini IA. Biochar Chemistry in a Weathered Tropical Soil: Kinetics of Phosphorus Sorption. Agriculture. 2021; 11(4):295. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11040295
Chicago/Turabian StyleMorales, Marina Moura, Nicholas Brian Comerford, Maurel Behling, Daniel Carneiro de Abreu, and Iraê Amaral Guerrini. 2021. "Biochar Chemistry in a Weathered Tropical Soil: Kinetics of Phosphorus Sorption" Agriculture 11, no. 4: 295. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11040295