Ergot Alkaloid Contents in Hybrid Rye are Reduced by Breeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trials and Inoculation Procedure
2.2. Sample Preparation
2.3. High Performance Liquid Chromatography (HPLC) Analysis of EAs
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) of EAs
2.5. Statistical Analyses
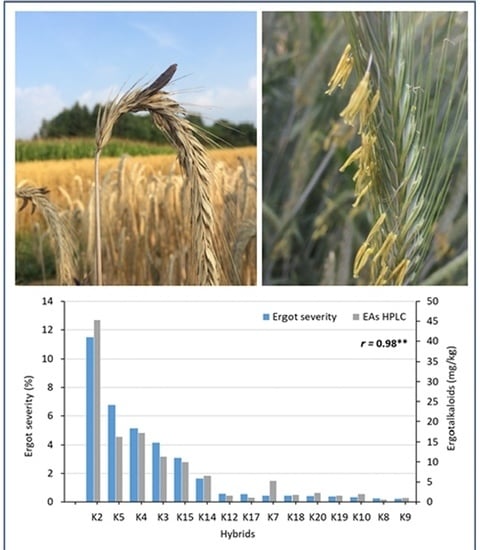
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wegulo, S.N.; Carlson, M.P. Ergot of Small Grain Cereals and Grasses and its Health Effects on Humans and Livestock. 2011. University of Nebraska, Extension, EC1880. Available online: http://ianrpubs.unl.edu/live/ec1880/build/ec1880.pdf (accessed on 9 March 2021).
- Pažoutová, S. The evolutionary strategy of Claviceps. In Clavicipitalean Fungi: Evolutionary Biology, Chemistry, Biocontrol and Cultural Impacts; White, F., Bacon, C.W., Hywel-Jones, N.L., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 329–354. [Google Scholar]
- Schumann, G.L.; Uppala, S. Ergot of rye. The Plant Health Instructor. 2000. Updated 2017. Available online: https://www.apsnet.org/edcenter/disandpath/fungalasco/pdlessons/Pages/Ergot.aspx (accessed on 10 February 2021).
- Schiff, P.L. Ergot and its alkaloids. Am. J. Pharm. Educ. 2006, 70, 98. [Google Scholar] [CrossRef]
- Hulvová, H.; Galuszka, P.; Frébortová, J.; Frébort, I. Parasitic fungus Claviceps as a source for biotechnological production of ergot alkaloids. Biotechnol. Adv. 2013, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Geiger, H.H. Biology, genetics, and management of ergot (Claviceps spp.) in rye, sorghum, and pearl millet. Toxins 2015, 7, 659–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, H.H.; Morgenstern, K. Applied genetic studies oncytoplasmic pollen sterility in winter rye (Angewandt-genetische Studien zur cytoplasmatischen Pollensterilität bei Winterroggen). Theor. Appl. Genet. 1975, 46, 269–276. (In German) [Google Scholar] [CrossRef] [PubMed]
- Geiger, H.H.; Yuan, Y.; Miedaner, T.; Wilde, P. Environmental sensitivity of cytoplasmic genic male sterility (CMS) in Secale cereale L. In Genetic Mechanisms for Hybrid Breeding (Advances in Plant Breeding, 18); Kück, U., Wricke, G., Eds.; Paul Parey Scientific Publishers: Berlin, Germany, 1995; pp. 7–17. [Google Scholar]
- Miedaner, T.; Wilde, P.; Wortmann, H. Combining ability of non-adapted sources for male-fertility restoration in Pampa CMS of hybrid rye. Plant Breed. 2005, 124, 39–43. [Google Scholar] [CrossRef]
- BSL (Beschreibende Sortenliste) Cereals, Maize, Oil and Fiber Plants, Legumes, Beets, Cover Crops [Getreide, Mais, Öl- und Faserpflanzen, Leguminosen, Rüben, Zwischenfrüchte] (In German). Hannover. 2020. Available online: https://www.bundessortenamt.de/bsa/sorten/beschreibende-sortenlisten/download-bsl-im-pdf-format (accessed on 28 March 2021).
- Schwake-Anduschus, C.; Lorenz, N.; Lahrssen-Wiederholt, M.; Lauch, A.; Dänicke, S. German monitoring 2012–2014: Ergot of Claviceps purpurea and ergot alkaloids (EA) in feeding stuffs and their toxicological relevance for animal feeding. J. Consum. Prot. Food Saf. 2020, 15, 321–329. [Google Scholar] [CrossRef]
- Raditsching, A.; Austrian Agency for Health and Food Safety (AGES), Institute for Food Safety, Linz, Austria. Personal communication, 2020.
- European Food Safety Authority (EFSA). Scientific Opinion on Ergot Alkaloids in Food and Feed. EFSA Panel on Contaminants in the Food Chain (CONTAM). 2012. EFSA 10: 2798. Available online: http://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/10.2903/j.efsa.2012.2798/pdf (accessed on 12 March 2021).
- Miedaner, T.; Mirdita, V.; Rodemann, B.; Drobeck, T.; Rentel, D. Genetic variation of winter rye cultivars for their ergot (Claviceps purpurea) reaction tested in a field design with minimized interplot interference. Plant Breed. 2010, 129, 58–62. [Google Scholar] [CrossRef]
- Miedaner, T.; Dänicke, S.; Schmiedchen, B.; Wilde, P.; Wortmann, H.; Dhillon, B.S.; Mirdita, V. Genetic variation for ergot (Claviceps purpurea) resistance and alkaloid concentrations in cytoplasmic-male sterile winter rye under pollen isolation. Euphytica 2010, 173, 299–306. [Google Scholar] [CrossRef]
- Kirchhoff, H. Contributions to the biology and physiology of the ergot fungus [Beiträge zur Biologie und Physiologie des Mutterkornpilzes] (In German). Centralbl. Bakteriol. Parasitenk. Abt. II 1929, 77, 310–369. [Google Scholar]
- Kodisch, A.; Oberforster, M.; Raditschnig, A.; Rodemann, B.; Tratwal, A.; Danielewicz, J.; Korbas, M.; Schmiedchen, B.; Eifler, J.; Gordillo, A.; et al. Covariation of ergot severity and alkaloid content measured by HPLC and one ELISA Method in inoculated winter rye across three isolates and three European countries. Toxins 2020, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH Monograph. 2001. Available online: https://www.julius-kuehn.de/media/Veroeffentlichungen/bbch%20epaper%20en/page.pdf (accessed on 4 March 2021).
- BVL L 15.01/02–5:2012–01. Investigation of food-determination of ergot alkaloids in rye and wheat-HPLC method with purification on a basic aluminum oxide solid phase [Untersuchung von Lebensmitteln—Bestimmung von Ergotalkaloiden in Roggen und Weizen—HPLC-Verfahren mit Reinigung an einer basischen Aluminiumoxid-Festphase]. 2012. Available online: https://www.beuth.de/de/technische-regel/bvl-l-15-01-02-5/150736503 (accessed on 4 June 2021). (In German).
- Bernal-Vasquez, A.M.; Utz, H.F.; Piepho, H.P. Outlier detection methods for generalized latt ices: A case study on the transition from ANOVA to REML. Theor. Appl. Genet. 2016, 129, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G.; Cox, G.M. Experimental Designs; Wiley: New York, NY, USA, 1957; ISBN 978-0-471-54567-5. [Google Scholar]
- Fehr, W.R. Principles of Cultivar Development, Theory and Technique; Macmillan: New York, NY, USA, 1987; Volume 1, ISBN 0029499208. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3–900051–07–0. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2016; Available online: https://www.rstudio.com/ (accessed on 4 March 2021).
- Alderman, S. Ergot: Biology and Control. 2006. Available online: https://www.ars.usda.gov/SP2UserFiles/person/81/ErgotDVDtranscript.pdf (accessed on 26 March 2021).
- Engelke, T. Approaches for an Integrated Control of Ergot (Claviceps purpurea [Fr.] Tul.) in Rye [Ansätze für eine integrierte Bekämpfung des Mutterkorns (Claviceps purpurea [Fr.] Tul.) im Roggen]. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2002. (In German). [Google Scholar]
- Miedaner, T.; Wilde, P. Selection strategies for disease-resistant hybrid rye. In Advances in Breeding Techniques for Cereals; Ordon, F., Friedt, W., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 223–246. ISBN 978-1786762443. [Google Scholar]
- Kodisch, A.; Wilde, P.; Schmiedchen, B.; Fromme, F.J.; Rodemann, B.; Tratwal, A.; Oberforster, M.; Wieser, F.; Schiemann, A.; Jørgensen, L.N.; et al. Ergot infection in winter rye hybrids shows differential contribution of male and female genotypes and environment. Eupyhtica 2020, 216, 65. [Google Scholar] [CrossRef] [Green Version]
- Blaney, B.J.; Molloy, J.B.; Brock, I.J. Alkaloids in Australian rye ergot (Claviceps purpurea) sclerotia: Implications for food and stockfeed regulations. Anim. Prod. Sci. 2009, 49, 975–982. [Google Scholar] [CrossRef]
- Kniel, B.; Meißner, M.; Koehler, P.; Schwake-Anduschus, C. Studies on the applicability of HPLC-FLD and HPLC–MS/MS for the determination of ergot alkaloids in rye-containing breads. J. Consum. Prot. Food Saf. 2018, 13, 69–78. [Google Scholar] [CrossRef]
- Müller, C.; Kemmlein, S.; Klaffke, H.; Krauthaus, W.; Preiß-Weigert, A.; Wittkowski, R. A basic tool for risk assessment: A new method for the analysis of ergot alkaloids in rye and selected rye products. Mol. Nutr. Food Res. 2009, 53, 500–550. [Google Scholar] [CrossRef] [PubMed]
- Tittlemier, S.A.; Drul, D.; Roscoe, M.; McKendry, T. Occurrence of ergot and ergot alkaloids in western Canadian wheat and other cereals. J. Agric. Food Chem. 2015, 63, 6644–6650. [Google Scholar] [CrossRef] [PubMed]
- Agrarmeteorologie Baden-Württemberg. Available online: https://www.wetter-bw.de (accessed on 10 March 2021).
- Dhillon, B.S.; Mirdita, V.; Miedaner, T. Preliminary evaluation of locations for conducting selection for resistance to ergot (Claviceps purpurea) in rye. Indian J. Plant Genet. Resour. 2010, 23, 265–268. [Google Scholar]
- Veršilovskis, A.; Fauw, D.P.K.H.P.; Smits, N.; Mulder, P.P.J.; Mol, H.; de Nijs, M. Screening of Ergot Alkaloids by ELISA Test. Kits available on the Market. EURL Mycotoxins and Plant Toxins; Wageningen Food Safety Research, Part of Wageningen University & Research: Wageningen, The Netherlands, 2019; EURL-MP-report_001; Available online: https://www.wur.nl/en/show/EURL-MP-report_001-Screening-of-ergot-alkaloids-using-ELISA-kits.htm (accessed on 12 March 2021).
- Vendelbo, N.; Mahmood, K.; Sarup, P.; Kristensen, P.; Orabi, J.; Jahoor, A. Genetic architecture of male fertility restoration in a hybrid breeding system of rye (Secale cereale L.). Res. Sq. 2021. reprinted in Res. Sq. 2021. Available online: https://assets.researchsquare.com/files/rs-275908/v1_stamped.pdf (accessed on 31 March 2021). [CrossRef]
- Kodisch, A.; Schmiedchen, B.; Eifler, J.; Gordillo, A.; Siekmann, D.; Fromme, F.J.; Oberforster, M.; Miedaner, T. Maternal differences for the reaction to ergot in unfertilized hybrid rye (Secale cereale). Eur. J. Plant. Pathol. 2021. submitted. [Google Scholar]


| Female | Male Line | |||||
|---|---|---|---|---|---|---|
| Line | PE2 | PE3 | PE4 | PE5 | Mean | Sign. a |
| Anther score (1–9): | ||||||
| SE2 | 8.25 | 8.25 | 7.75 | 2.00 | 6.56 | a |
| SE3 | 8.00 | - | 7.50 | 3.00 | 6.17 | a |
| SE4 | 7.75 | 4.75 | 7.25 | 1.50 | 5.31 | b |
| SE5 | 7.75 | 4.00 | 7.75 | 1.75 | 5.31 | b |
| Mean | 7.94 | 5.67 | 7.56 | 2.06 | 5.84 | |
| Sign. a | a | b | a | c | ||
| Ergot severity (%): | ||||||
| SE2 | 0.44 | 0.57 | 0.54 | 11.47 | 3.26 | a |
| SE3 | 0.25 | - | 0.43 | 4.16 | 1.61 | b |
| SE4 | 0.22 | 1.63 | 0.37 | 5.12 | 1.84 | b |
| SE5 | 0.31 | 3.09 | 0.39 | 6.76 | 2.64 | ab |
| Mean | 0.31 | 1.76 | 0.43 | 6.88 | 2.34 | |
| Sign. a | a | b | a | c | ||
| EAs HPLC (mg/kg): | ||||||
| SE2 | 5.25 | 1.51 | 1.02 | 45.27 | 13.26 | a |
| SE3 | 0.57 | - | 1.72 | 11.28 | 4.52 | c |
| SE4 | 0.92 | 6.48 | 1.54 | 17.27 | 6.55 | b |
| SE5 | 1.91 | 9.99 | 2.25 | 16.35 | 7.63 | b |
| Mean | 2.16 | 5.99 | 1.63 | 22.54 | 7.99 | |
| Sign. a | a | b | a | c | ||
| EAs ELISA (mg/kg): | ||||||
| SE2 | 32.64 | 18.30 | 28.40 | 75.82 | 38.79 | a |
| SE3 | 20.94 | - | 43.67 | 133.09 | 65.90 | b |
| SE4 | 22.13 | 51.29 | 27.28 | 126.99 | 56.92 | ab |
| SE5 | 55.85 | 112.99 | 27.06 | 88.48 | 71.10 | b |
| Mean | 32.89 | 60.86 | 31.60 | 106.10 | 58.18 | |
| Sign. a | a | a | a | b | ||
| All Environments (n = 11) | Test Environments (n = 2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Ergot Severity a | Anther Score | Ergot Severity a | Anther Score | EA Content HPLC a | EA Content ELISA a | ||||||
| Variance components: | ||||||||||||
| GCA male (M) | 13.07 | *** | 271.98 | *** | 13.39 | *** | 115.5 | *** | 1429 | *** | 17612 | *** |
| GCA female (F) | 0.37 | ** | 5.64 | *** | 0.38 | *** | 6.08 | ** | 182 | *** | 3849 | * |
| SCA | 0.25 | ** | 4.04 | *** | 0.57 | *** | 3.69 | ** | 242 | *** | 2845 | * |
| M × environment (E) | 1.32 | *** | 17.44 | *** | 0.68 | *** | 0.06 | 831 | *** | 3586 | * | |
| F × E | 0.17 | ** | 1.13 | 0.16 | * | 0.58 | 158 | *** | 3243 | |||
| F × M × E | 0.14 | ** | 1.26 | * | 0.15 | * | 1.25 | 190 | *** | 3032 | * | |
| Error | 0.08 | 0.83 | 0.05 | 1.01 | 5 | 1159 | ||||||
| 0.87 | 0.89 | 0.95 | 0.88 | 0.98 | 0.8 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miedaner, T.; Kodisch, A.; Raditschnig, A.; Eifler, J. Ergot Alkaloid Contents in Hybrid Rye are Reduced by Breeding. Agriculture 2021, 11, 526. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11060526
Miedaner T, Kodisch A, Raditschnig A, Eifler J. Ergot Alkaloid Contents in Hybrid Rye are Reduced by Breeding. Agriculture. 2021; 11(6):526. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11060526
Chicago/Turabian StyleMiedaner, Thomas, Anna Kodisch, Armin Raditschnig, and Jakob Eifler. 2021. "Ergot Alkaloid Contents in Hybrid Rye are Reduced by Breeding" Agriculture 11, no. 6: 526. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11060526