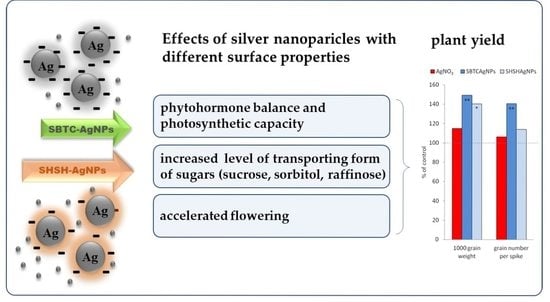
Silver Nanoparticles and Silver Ions Differentially Affect the Phytohormone Balance and Yield in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. AgNP Preparation and Characteristics
2.2. Experimental Design
2.3. Slow Kinetic Fluorescence of Chlorophyll a
2.4. Soluble Sugars Profiling
2.5. Estimation of Endogenous Phytohormones
2.6. SS (EC 2.4.1.13) and SPS (EC 2.4.1.14) Activity
2.7. Statistical Analysis of the Results
3. Results
3.1. Influence of the Surface Properties of Charge-Stabilized AgNPs and Silver Ions on Height of Plants, Phytohormone Balance and Time of Flowering
3.2. Influence of the Surface Properties of Charge-Stabilized AgNPs and Silver Ions on Photosynthesis, Soluble Carbohydrates, SPS and SS Activity and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Yada, R. Nanotechnologies in agriculture: New tools for sustainable development. Trends Food Sci. Technol. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [Green Version]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the Challenges of Abiotic Stress in Plants: New Dimensions in the Field Application of Nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Arruda, S.C.C.; Silva, A.L.D.; Galazzi, R.M.; Azevedo, R.A.; Arruda, M.A.Z. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Shweta Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake translocation accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 2012, 7, e47674. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J.J. Phytostimulation of Poplars and Arabidopsis Exposed to Silver Nanoparticles and Ag+ at Sublethal Concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef]
- Gorczyca, A.; Pociecha, E.; Kasprowicz, M.J.; Niemiec, M. Effect of nanosilver in wheat seedlings and Fusarium culmorum culture systems. Eur. J. Plant Pathol. 2015, 142, 251–261. [Google Scholar] [CrossRef]
- Vinković, T.; Štolfa-Čamagajevac, I.; Tkalec, M.; Goessler, W.; Domazet-Jurašin, D.; Vinković-Vrček, I. Does plant growing condition affects biodistribution and biological effects of silver nanoparticles? Span. J. Agric. Res. 2019, 16, e0803. [Google Scholar] [CrossRef]
- Gray, W.M. Hormonal regulation of plant growth and development. PLoS Biol. 2004, 9, 1270–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarkowská, D.; Strnad, M. Plant ecdysteroids, plant sterols with intriguing distributions biological effects and relations to plant hormones. Planta 2016, 244, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, G.; Qian, H.; Liu, P.; Zhao, P.; Hu, Y. Effects of nano-TiO2 on photosynthetic characteristics of Ulmus elongata seedlings. Environ. Pollut. 2013, 176, 63–70. [Google Scholar] [CrossRef]
- Govindjee. Chlorophyll fluorescence. In Chlorophyll a Fluorescence: A Signature of Photosynthesis, 1st ed.; Papageorgiou, G.C., Govindjee, Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–42. [Google Scholar]
- Bilger, W.; Björkman, O. Temperature-dependence of violaxanthin deepoxidation and nonphotochemical fluorescence quenching in intact to leaves Gossypium hirsutum L. & Malva parviflora L. Planta 1991, 184, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Counce, P.A.; Gravois, K.A. Sucrose synthase activity as a potential indicator of high rice grain yield. Crop Sci. 2006, 46, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
- Pommerrenig, B.; Papini-Terzi, F.S.; Sauer, N. Differential regulation of sorbitol and sucrose loading into the phloem of Plantago major in response to salt stress. Plant Physiol. 2007, 144, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Oćwieja, M.; Adamczyk, Z. Monolayers of silver nanoparticles obtained by chemical reduction methods. Surf. Innov. 2014, 2, 160–172. [Google Scholar] [CrossRef]
- Barbasz, A.; Czyżowska, A. Is there a way to protect human immune cells against nanocytotoxicity? Mol. Simulat. 2019, 45, 585–594. [Google Scholar] [CrossRef]
- Oćwieja, M.; Adamczyk, Z.; Morga, M.; Michna, A. High density silver nanoparticle monolayers produced by colloid self-assembly on polyelectrolyte supporting layers. J. Colloid Interface Sci. 2011, 364, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Oćwieja, M.; Mrowiec, H.; Walas, S.; Lupa, D. Oxidative dissolution of silver nanoparticles: A new theoretical approach. J. Colloid Interface Sci. 2016, 469, 355–364. [Google Scholar] [CrossRef]
- Gorczyca, A.; Przemieniecki, S.W.; Kurowski, T.; Oćwieja, M. Early plant growth and bacterial community in rhizoplane of wheat and flax exposed to silver and titanium dioxide nanoparticles. Environ. Sci. Pollut. Res. 2018, 25, 33820–33826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochem. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a non intrusive indicator for rapid assessment of in vivo photosynthesis. In Excophysiology of Photosynthesis; Schultze, E.-D., Caldwell, M.M., Eds.; Springer: Berlin, Germany, 1994; p. 4970. [Google Scholar] [CrossRef]
- Pociecha, E.; Dziurka, M. Trichoderma interferes with cold acclimation by lowering soluble sugars accumulation resulting in reduced pink snow mould (Microdochium nivale) resistance of winter rye. Environ. Exp. Bot. 2015, 109, 193–200. [Google Scholar] [CrossRef]
- Hura, T.; Dziurka, M.; Hura, K.; Ostrowska, A.; Dziurka, K.; Gadzinowska, J. Wheat and rye genome confer specific phytohormone profile features and interplay under water stress in two phenotypes of triticale. Plant Physiol. Biochem. 2017, 118, 494–509. [Google Scholar] [CrossRef]
- Płażek, A.; Dubert, F.; Kopeć, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Waligórski, P.; Wolko, B. Long-Term Effects of Cold on Growth; Development and Yield of Narrow-Leaf Lupine May Be Alleviated by Seed Hydropriming or Butenolide. Int. J. Mol. Sci. 2018, 19, 2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalt-Torres, W.; Huber, S.C. Diurnal changes in Maize leaf photosynthesis. Plant Physiol. 1987, 83, 294–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.J.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Singh Brar, R.; Kumar, A.; Kaur, S. Impact of metal oxide nanoparticles on cotton (Gossypium hirsutum L.): A physiological perspective. J. Cotton Res. 2021, 4, 16. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.J. (Ed.) The plant hormones: Their nature, occurrence, and functions. In Plant Hormones, 3rd ed.; XIV,802; Springer: Dordrecht, The Netherlands, 2010; pp. 1–15. [Google Scholar] [CrossRef]
- King, R.W.; Mander, L.N.; Asp, T.; MacMillan, C.P.; Blundell, C.A.; Evans, L.T. Selective Deactivation of Gibberellins below the Shoot Apex is Critical to Flowering but Not to Stem Elongation of Lolium. Mol. Plant 2008, 1, 295–307. [Google Scholar] [CrossRef] [PubMed]
- King, R.W.; Evans, L.T.; Mander, L.M.; Moritz, T.; Pharis, R.P.; Twitchin, B. Synthesis of gibberellin GA6 and examination of its role in flowering of Lolium temulentum. Phytochemistry 2003, 62, 77–82. [Google Scholar] [CrossRef]
- Schäfer, M.; Brütting, C.; Meza-Canales, I.D.; Großkinsky, D.K.; Vankova, R.; Baldwin, I.T.; Meldau, S. The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J. Exp. Bot. 2015, 66, 4873–4884. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A. Wheat. In Potential Productivity of Field Crops under Different Environments; Smith, W.H., Banta, J.J., Eds.; IRRI: Los Baños, CA, USA, 1984; pp. 129–154. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis, Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; Chapter 25; pp. 443–480. [Google Scholar]
- Osmond, C.B. What is photoinhibition? Some insights from comparisons of shade and sun plants. In Photoinhibition of Photosynthesis, from Molecular Mechanisms to the Field; Baker, N.R., Bowyer, J.R., Eds.; Bios Scientific Publishers: Oxford, UK, 1994; pp. 1–24. [Google Scholar]
- Pociecha, E.; Dziurka, M.; Waligórski, P.; Krępski, T.; Janeczko, A. 24-Epibrassinolide pre-treatment modifies cold-induced photosynthetic acclimation mechanisms and phytohormone response of perennial ryegrass in cultivar-dependent manner. J. Plant Growth Regul. 2017, 36, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Barber, J.; Andersson, B. Too much of a good thing, light can be bad for photosynthesis. Trends Biochem. Sci. 1992, 12, 61–66. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Huber, S.C.; Huber, J.L. Role and regulation of sucrose-phosphate synthase in higher plants. Ann. Rev. Plant Biol. 1996, 47, 431–444. [Google Scholar] [CrossRef]
- Winter, H.S.; Huber, C. Regulation of Sucrose Metabolism in Higher Plants, Localization and Regulation of Activity of Key Enzymes. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 253–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Q.; Wen, X.; Lu, C. Enhanced Sucrose Loading Improves Rice Yield by Increasing Grain Size. Plant Physiol. 2015, 169, 2848–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Ma, M.; Lu, H.; Meng, Q.; Li, G.; Yang, X. Photosynthesis sucrose metabolism and starch accumulation in two NILs of winter wheat. Photosynth. Res. 2015, 126, 363–373. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef] [PubMed]
- Eichert, T.; Goldbach, H.E. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces—Further evidence for a stomatal pathway. Physiol. Plant. 2008, 132, 491–502. [Google Scholar] [CrossRef]





| Control | AgNO3 | SBTCAgNPs | SHSHAgNPs | ||
|---|---|---|---|---|---|
| tZ | 3 | 29.35 ± 5.99 a | 30.59 ± 4.32 a | 22.44 ± 1.14 b | 28.63 ± 1.90 ab |
| 4 | 7.63 ± 1.34 c | 7.65 ± 1.93 c | 6.05 ± 0.43 c | 5.90 ± 1.38 c | |
| 5 | 4.21 ± 0.6 c | 3.74 ± 0.62 c | 4.84 ± 0.79 c | 4.62 ± 0.11 c | |
| cZ | 3 | 117.89 ± 7.25 a | 116.06 ± 11.00 a | 52.19 ± 6.52 bc | 85.08 ± 3.13 ab |
| 4 | 114.19 ± 25.61 a | 103.96 ± 34.13 a | 42.56 ± 9.82 cd | 48.97 ± 4.41 bc | |
| 5 | 13.66 ± 3.01 cd | 15.00 ± 4.46 cd | 7.32 ± 1.69 d | 6.74 ± 0.39 d | |
| K | 3 | nd | 3.52 ± 0.47 a | 4.28 ± 0.34 a | 3.94 ± 0.03 a |
| 4 | 3.48 ± 1.38 a | 2.41 ± 0.01 a | 3.67 ± 0.01 a | 3.36 ± 0.01 a | |
| 5 | 4.52 ± 0.01 a | 3.07 ± 0.01 a | 4.55 ± 0.40 a | 4.64 ± 0.49 a | |
| tZ(R) | 3 | 12.57 ± 5.06 a | 16.28 ± 0.24 a | 13.79 ± 1.79 a | 16.72 ± 1.85 a |
| 4 | 3.73 ± 0.16 b | 3.90 ± 1.29 b | 2.20 ± 0.40 b | 1.84 ± 0.28 b | |
| 5 | 1.30 ± 0.01 b | 1.50 ± 0.01 b | 1.58 ± 0.09 b | 1.60 ± 0.14 b | |
| cZ(R) | 3 | 698.17 ± 107.64 a | 668.32 ± 79.37 ab | 203.12 ± 21.83 d | 510.48 ± 58.08 c |
| 4 | 574.12 ± 25.42 abc | 535.35 ± 52.70 bc | 103.78 ± 10.01 de | 203.49 ± 17.95 d | |
| 5 | 20.64 ± 7.10 e | 29.35 ± 11.59 e | 9.19 ± 2.72 e | 17.72 ± 5.58 e | |
| GA4 | 3 | 5.26 ± 1.47 bcd | 8.00 ± 1.27 ab | 8.79 ± 1.10 a | 7.07 ± 0.91 abc |
| 4 | 2.96 ± 0.87 d | 4.77 ± 1.42 bcd | 4.69 ± 1.11 bcd | 3.88 ± 1.37 cd | |
| 5 | 3.23 ± 1.08 d | 4.05 ± 0.77 cd | 2.71 ± 0.09 d | 3.25 ± 0.63 d | |
| GA3 | 3 | 41.61 ± 5.58 a | 16.71 ± 9.56 a | 32.67 ± 0.01 a | 33.83 ± 6.75 a |
| 4 | 55.85 ± 26.05 a | 35.83 ± 12.29 a | 37.07 ± 16.26 a | 31.52 ± 0.01 a | |
| 5 | 27.75 ± 7.78 a | 17.77 ± 3.00 a | 24.38 ± 8.95 a | 30.83 ± 0.19 a | |
| GA1 | 3 | 26.08 ± 5.86 ab | 16.67 ± 1.50 b | 21.43 ± 2.73 b | 24.54 ± 3.31 ab |
| 4 | 35.02 ± 0.01 a | 18.34 ± 1.04 b | 16.99 ± 1.25 b | 19.64 ± 3.43 b | |
| 5 | 22.29 ± 1.64 b | 16.77 ± 1.16 b | 22.00 ± 4.49 b | 22.00 ± 2.14 b | |
| GA6 | 3 | 454.30 ± 40.10 b | 652.73 ± 124.22 b | 494.16 ± 83.39 b | 704.15 ± 68.94 b |
| 4 | 677.67 ± 120.75 b | 731.55 ± 161.38 b | 614.24 ± 156.35 b | 798.03 ± 103.83 b | |
| 5 | 1513.78 ± 88.50 a | 1417.05 ± 135.90 a | 1443.15 ± 353.70 a | 1314.17 ± 86.25 a | |
| ABA | 3 | 919.41 ± 34.33 cd | 195.15 ± 42.98 d | 683.67 ± 92.59 bcd | 562.35 ± 56.66 bcd |
| 4 | 423.79 ± 173.62 cd | 629.44 ± 17.42 bcd | 664.69 ± 115.39 bcd | 749.77 ± 117.54 bcd | |
| 5 | 843.01 ± 44.67 cd | 620.632 ± 80.67 bcd | 1561.19 ± 230.69 a | 1142.39 ± 182.83 ab | |
| JA | 3 | 3280.87 ± 434.59 b | 784.73 ± 164.89 c | 8456.72 ± 358.65 a | 744.84 ± 137.38 c |
| 4 | 71.70 ± 25.84 c | 492.02 ± 15.00 c | 223.47 ± 59.61 c | 8782.62 ± 728.12 a | |
| 5 | 65.12 ± 7.97 c | 276.96 ± 8.71 c | 303.12 ± 52.65 c | 179.31 ± 22.17 c | |
| IAA | 3 | 65.53 ± 14.89 ab | 51.04 ± 16.41 abc | 82.53 ± 6.37 a | 47.20 ± 34.36 abc |
| 4 | 36.36 ± 7.34 bc | 19.65 ± 3.38 c | 46.54 ± 12.52 abc | 37.18 ± 1.59 bc | |
| 5 | 32.41 ± 4.01 bc | 53.51 ± 11.41 abc | 25.48 ± 5.80 bc | 20.86 ± 3.38 c |
| Week | Glucose | Fructose | Sucrose | Sorbitol | Rafinose | Maltose | Kestose | |
|---|---|---|---|---|---|---|---|---|
| CONTROL | 3 | 25.01 ± 4.63 bcd | 20.12 ± 3.77 bc | 22.38 ± 3.38 c | nd | 0.99 ± 0.1 c | 3.16 ± 0.61 c | 1.90 ± 0.3 e |
| 4 | 41.13 ± 6.15 a | 32.21 ± 5.34 a | 36.96 ± 7.39 c | nd | 1.67 ± 0.2 bc | 3.95 ± 0.49 c | 5.80 ± 0.94 d | |
| 5 | 18.46 ± 2.48 cd | 21.05 ± 2.5 bc | 60.24 ± 7.56 b | 13.74 ± 1.54 b | 1.67 ± 0.98 bc | 4.59 ± 0.68 bc | 8.14 ± 0.56 cd | |
| AgNO3 | 3 | 27.40 ± 4.09 bcd | 22.14 ± 2.32 b | 20.01 ± 2.47 c | nd | 0.93 ± 15 c | 3.19 ± 0.33 c | 2.06 ± 0.29 e |
| 4 | 27.74 ± 4.41 bcd | 24.45 ± 3.2 b | 59.10 ± 6.1 b | 10.70 ± 0.1 bc | 1.30 ± 0.1 c | 4.19 ± 0.7 bc | 6.76 ± 2.17 d | |
| 5 | 16.60 ± 0.96 d | 18.38 ± 0.7 bc | 64.52 ± 4.7 b | 9.96 ± 0.2 c | 2.52 ± 0.1 ab | 7.06 ± 3.05 a | 12.91 ± 0.51 b | |
| SBTCAgNPs | 3 | 17.95 ± 2.7 cd | 12.56 ± 2.23 c | 28.67 ± 10.07 c | nd | 1.01 ± 0.2 c | 3.40 ± 0.48 c | 2.27 ± 0.44 e |
| 4 | 28.57 ± 2.06 bc | 19.82 ± 1.34 bc | 97.79 ± 6.51 a | 0.98 ± 0.11 d | 1.31 ± 0.28 c | 4.02 ± 0.12 c | 6.47 ± 1.06 d | |
| 5 | 20.64 ± 1.98 cd | 20.22 ± 0.91 bc | 102.94 ± 6.25 a | 19.25 ± 1.31 a | 2.74 ± 0.27 a | 4.79 ± 0.53 bc | 10.94 ± 0.22 bc | |
| SHSHAgNPs | 3 | 22.13 ± 2.23 bcd | 18.68 ± 1.2 bc | 27.73 ± 2.78 c | nd | 0.92 ± 0.05 c | 3.39 ± 0.2 c | 1.68 ± 0.13 e |
| 4 | 32.73 ± 2.78 ab | 21.04 ± 0.76 bc | 83.63 ± 6.77 a | 0.64 ± 0.04 d | 1.21 ± 0.04 c | 3.53 ± 0.14 c | 4.96 ± 0.82 de | |
| 5 | 16.88 ± 2.43 d | 17.74 ± 1.77 bc | 94.96 ± 1.07 a | 17.75 0.08 a | 2.39 ± 0.52 ab | 6.14 ± 0. 16 ab | 16.50 ± 0.67 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pociecha, E.; Gorczyca, A.; Dziurka, M.; Matras, E.; Oćwieja, M. Silver Nanoparticles and Silver Ions Differentially Affect the Phytohormone Balance and Yield in Wheat. Agriculture 2021, 11, 729. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11080729
Pociecha E, Gorczyca A, Dziurka M, Matras E, Oćwieja M. Silver Nanoparticles and Silver Ions Differentially Affect the Phytohormone Balance and Yield in Wheat. Agriculture. 2021; 11(8):729. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11080729
Chicago/Turabian StylePociecha, Ewa, Anna Gorczyca, Michał Dziurka, Ewelina Matras, and Magdalena Oćwieja. 2021. "Silver Nanoparticles and Silver Ions Differentially Affect the Phytohormone Balance and Yield in Wheat" Agriculture 11, no. 8: 729. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture11080729