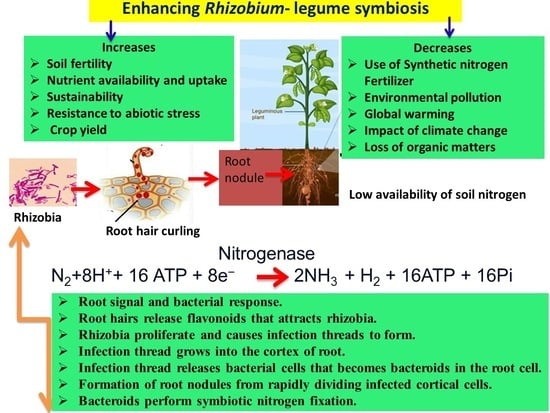
Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change
Abstract
:1. Introduction
1.1. Nitrogen Resources: Tackling Protein Scarcity Globally
1.2. Crop Production and Environmental Consequences of Nitrogen Fertilizer Usage
2. Biological Nitrogen Fixation Systems
Rhizobium–Legume Symbiotic Relationship and Environmental Stress
3. Effects of N Fertilizer on Rhizobium–Legume Molecular Signaling
3.1. Isoflavonoids
3.2. Nod Factors
3.3. Nodulation Receptor Kinases (NORKs)
3.4. Calcium Spikes Play a Crucial Role in Symbiotic Signaling
3.5. Cytokinins and Auxins
3.6. Reactive Oxygen Species (ROS)
4. Effects of N Fertilizer on Rhizobial Motility
5. Effect of N Fertilizer on Root-Hair Curling, Infection Thread Formation and Nodulation
6. Effect of Nitrogen Fertilizers on Nodule Physiology
6.1. Nodule Nitrate Reductase
6.2. Leghemoglobin
6.3. Nitrogenase Activity
7. Mitigation Strategies
8. Future Prospects
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semba, R.D. The Rise and Fall of Protein Malnutrition in Global Health. Ann. Nutr. Metab. 2016, 69, 79–88. [Google Scholar] [CrossRef]
- Webb, P.; Stordalen, G.A.; Singh, S.; Wijesinha-Bettoni, R.; Shetty, P.; Lartey, A. Hunger and malnutrition in the 21st century. BMJ 2018, 361, k2238. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Parisi, G.; Tulli, F.; Fortina, R.; Marino, R.; Bani, P.; Zotte, A.D.; De Angelis, A.; Piccolo, G.; Pinotti, L.; Schiavone, A.; et al. Protein hunger of the feed sector: The alternatives offered by the plant world. Ital. J. Anim. Sci. 2020, 19, 1204–1225. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050? Rome, FAO. 2009. Available online: http://www.fao.org/fileadmin/tempelates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 5 January 2018).
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and Genetic Approaches Improving Nitrogen Use Efficiency in Cereal Crops: A Review. Front. Plant Sci. 2021, 12, 657629. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition Through Plant-Based Foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef]
- Bernhard, A. The nitrogen cycle: Processes, players, and human impact. Nat. Educ. Knowl. 2010, 3, 25. [Google Scholar]
- Habete, A.; Buraka, T. Effect of Rhizobium inoculation and nitrogen fertilization on nodulation and yield response of common bean (Phaseolus vulgaries L.) at Boloso Sore, Southern Ethiopia. J. Biol. Agric. Health 2016, 6, 72–75. [Google Scholar]
- Aboelfadel, M.; Hassan, G.; Taha, M.A. Impact of Nitrogen Fertilization Types on Leaf Miner, Liriomyza trifolii Infestation, Growth and Productivity of Pea Plants under Pest Control Program. J. Adv. Agric. Res. 2023, 28, 92–105. [Google Scholar] [CrossRef]
- Vance, C.P. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol. 2001, 127, 390–397. [Google Scholar] [CrossRef]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Rulli, M.C.; Ali, S.; Chiarelli, D.D.; Dell’Angelo, J.; Mueller, N.D.; Scheidel, A.; Siciliano, G.; D’Odorico, P. Energy implications of the 21st century agrarian transition. Nat. Commun. 2021, 12, 2319. [Google Scholar] [PubMed]
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef] [PubMed]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9. [Google Scholar] [CrossRef]
- IFA. Energy Efficiency and CO2 Emissions in Ammonia Production. 2009. Available online: https://www.fertilizer.org/images/Library_Downloads/2009_IFA_energy_efficiency.pdf (accessed on 9 June 2018).
- Zhang, W.-F.; Dou, Z.-X.; He, P.; Ju, X.-T.; Powlson, D.; Chadwick, D.; Norse, D.; Lu, Y.-L.; Zhang, Y.; Wu, L.; et al. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc. Natl. Acad. Sci. USA 2013, 110, 8375–8380. [Google Scholar]
- Brentrup, F.; Hoxha, A.; Christensen, B. Carbon footprint analysis of mineral fertilizer production in Europe and other world regions. In Proceedings of the 10th International Conference on Life Cycle Assessment of Food (LCA Food 2016), Dublin, Ireland, 19–21 October 2016. [Google Scholar]
- Wood, S.; Cowie, A. A review of greenhouse gas emission factors for fertilizer production. IEA Bioenergy Task 2004, 38, 1–20. [Google Scholar]
- Snyder, C.; Bruulsema, T.; Jensen, T.; Fixen, P. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Taheripour, F.; Zhao, X.; Tyner, W.E. The impact of considering land intensification and updated data on biofuels land use change and emissions estimates. Biotechnol. Biofuels 2017, 10, 191. [Google Scholar] [CrossRef]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. 2006. Available online: https://www.osti.gov/etdeweb/biblio/20880391 (accessed on 19 September 2023).
- Statista 2023. Global Consumption of Agricultural Fertilizer from 1965 to 2020, by Nutrient (In Million Metric Tons). Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/438967/fertilizer-consumption-globally-by-nutrient/ (accessed on 19 September 2023).
- Woods, J.; Williams, A.; Hughes, J.K.; Black, M.; Murphy, R. Energy and the food system. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2991–3006. [Google Scholar]
- Gellings, C.W.; Parmenter, K.E. Energy Efficiency in Fertilizer Production and Use. Efficient Use and Conservation of Energy. In Encyclopedia of Life Support Systems; Gellings, C.W., Ed.; EPRI: Washington, DC, USA, 2016; pp. 123–136. [Google Scholar]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and their contaminants in soils, surface, and groundwater. Encycl. Anthr. 2018, 5, 225–240. [Google Scholar]
- Zheng, M.; Zhou, Z.; Luo, Y.; Zhao, P.; Mo, J. Global pattern and controls of biological nitrogen fixation under nutrient en-richment: A meta-analysis. Glob. Chang. Biol. 2019, 25, 3018–3030. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Are Nitrogen Fertil. Deleterious Soil Health? Agronomy 2018, 8, 48. [Google Scholar] [CrossRef]
- Sainju, U.M.; Ghimire, R.; Pradhan, G.P. Nitrogen Fertilization I: Impact on Crop, Soil, and Environment. In Nitrogen Fixation, Biochemistry; Rigobelo, R.C., Serra, A.B., Eds.; Intech Open Book: London, UK, 2019; Volume 11, pp. 69–92. [Google Scholar]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Sud, M. Managing the Biodiversity Impacts of Fertilizer and Pesticide Use: Overview and Insights from Trends and Policies across Selected OECD Countries; OECD Environment Working Papers, No. 155; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Ouikhalfan, M.; Lakbita, O.; Delhali, A.; Assen, A.H.; Belmabkhout, Y. Toward Net-Zero Emission Fertilizers Industry: Greenhouse Gas Emission Analyses and Decarbonization Solutions. Energy Fuels 2022, 36, 4198–4223. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2019, 295, 122223. [Google Scholar] [CrossRef]
- Aggani, S.L. Development of bio-fertilizers and its future perspective. Sch. Acad. J. Pharm. 2013, 2, 327–332. [Google Scholar]
- Chen, X.; Wang, Y.-H.; Ye, C.; Zhou, W.; Cai, Z.-C.; Yang, H.; Han, X. Atmospheric Nitrogen Deposition Associated with the Eutrophication of Taihu Lake. J. Chem. 2018, 2018, 4017107. [Google Scholar] [CrossRef]
- Herridge, D.F. Inoculation Technology for Legumes. In Nitrogen-Fixing Leguminous Symbioses (77–115); Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Davies-Barnard, T.; Friedlingstein, P.; Zaehle, S.; Bovkin, V.; Fan, Y.; Fisher, R.; Lee, H.; Peano, D.; Smith, B.; Warlind, D.; et al. Evaluating Terrestrial Biological Nitrogen Fixation in CMIP6 Earth System Models. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2020; p. B024-04. [Google Scholar]
- Boddey, R.M.; Jantalia, C.P.; Conceiãão, P.C.; Zanatta, J.A.; Bayer, C.; Mielniczuk, J.; Dieckow, J.; DOS Santos, H.P.; Denardin, J.E.; Aita, C.; et al. Carbon accumulation at depth in Ferralsols under zero-till subtropical agriculture. Glob. Chang. Biol. 2010, 16, 784–795. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Herridge, D.F.; Denton, M.D.; McDonald, G.K.; McNeill, A.M.; Long, W.; Farquharson, R.; Malcolm, B. A Nitrogen Reference Manual for the Southern Cropping Region; Grains Research and Development Corporation (GRDC): Canberra, Australia, 2020; Available online: https://hdl.handle.net/1959.11/31716 (accessed on 19 September 2023).
- Sprent, J.I.; Sprent, P. Nitrogen Fixing Organisms: Pure and Applied Aspects; Chapman and Hall: London, UK, 1990; Volume 256. [Google Scholar]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [PubMed]
- Brock, T.D.; Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms; Prentice-Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS ONE 2013, 9, e56329. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-J.; Wang, L.-L.; Li, Q.; Shang, Q.-M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Geddes, B.A.; Oresnik, I.J. The Mechanism of Symbiotic Nitrogen Fixation. In The Mechanistic Benefits of Microbial Symbionts; Springer: Cham, Germany, 2016; pp. 69–97. [Google Scholar]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Coba de la Pena, T.; Fedorova, E.; Pueyo, J.J.; Lucas, M.M. The symbiosome: Legume and rhizobia co-evolution toward a nitrogen-fixing organelle? Front. Plant Sci. 2018, 8, 2229. [Google Scholar]
- Sprent, J.I.; Gehlot, H.S. Nodulated legumes in arid and semi-arid environments: Are they important? Plant Ecol. Divers. 2010, 3, 211–219. [Google Scholar] [CrossRef]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Mng’Ong’O, M.E.; Ojija, F.; Aloo, B.N. The role of Rhizobia toward food production, food and soil security through microbial agro-input utilization in developing countries. Case Stud. Chem. Environ. Eng. 2023, 8, 100404. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Bagy, M.K.; El-enany, A.W.E.S.; Bashandy, S.R. Activation of Rhizobium tibeticum with flavonoids en-hances nodulation, nitrogen fixation, and growth of fenugreek (Trigonella foenumgraecum L.) grown in cobalt-polluted soil. Arch. Environ. Contam. Toxicol. 2014, 66, 303–315. [Google Scholar]
- Abd-Alla, M.H.; Issa, A.A.; Ohyama, T. Impact of harsh environmental conditions on nodule formation and dinitrogen fixation of legumes. Adv. Biol. Ecol. Nitrogen Fixat. 2014, 9, 1. [Google Scholar]
- Abd-Alla, M.H.; Nafady, N.A.; Bashandy, S.R.; Hassan, A.A. Mitigation of effect of salt stress on the nodulation, nitrogen fixation and growth of chickpea (Cicer arietinum L.) by triple microbial inoculation. Rhizosphere 2019, 10, 100148. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Wahab, A.A. Survival of Rhizobium leguminosarum biovar viceae subjected to heat, drought, and salinity in soil. Biol. Plant. 1995, 37, 131–137. [Google Scholar] [CrossRef]
- Wahab, A.M.A.; Zahran, H.H.; Abd-Alla, M.H. Root-hair infection and nodulation of four grain legumes as affected by the form and the application time of nitrogen fertilizer. Folia Microbiol. 1996, 41, 303–308. [Google Scholar] [CrossRef]
- Abdel Wahab, A.M.; Abd-Alla, M.H. Effect of different rates of N-fertilizers on nodulation, nodule activities and growth of two field grown cvs. of soybean. In Fertilizers and Environment, Proceedings of the International Symposium “Fertilizers and Environment”, Salamanca, Spain, 26–29 September 1994; Springer: Dordrecht, The Netherlands, 1996; pp. 89–93. [Google Scholar]
- Abdel Wahab, A.; Mand Abd-Alla, M.H. Nodulation and nitrogenase activity of Vicia faba and Glycine max in relation to rhizobia strain, form and level of combined nitrogen. Phyton 1995, 35, 77–187. [Google Scholar]
- Wahab, A.M.A.; Abd-Alla, M.H. Effect of form and level of applied nitrogen on nitrogenase and nitrate reductase activities in faba beans. Biol. Plant. 1995, 37, 57–64. [Google Scholar] [CrossRef]
- Abdel Wahab, A.M.; Abd-Alla, M.H. Effect of combined nitrogen on the structure of N2-fixing nodules in two legumes. In Nitrogen Fixation: Hundred Years after; Gustav Fischer Stuttgart: New York, NY, USA, 1988; Volume 535. [Google Scholar]
- Junior, M.A.L.; Lima, A.; Arruda, J.; Smith, D. Effect of root temperature on nodule development of bean, lentil and pea. Soil Biol. Biochem. 2005, 37, 235–239. [Google Scholar] [CrossRef]
- Zhang, F.; Smith, D.L. Application of genistein to inocula and soil to overcome low spring soil temperature inhibition of soybean nodulation and nitrogen fixation. Plant Soil 1997, 192, 141–151. [Google Scholar] [CrossRef]
- Zhang, F.; Pan, B.; Smith, D.L. Application of gibberellic acid to the surface of soybean seed (t Glycine max (L.) Merr.) and symbiotic nodulation, plant development, final grain and protein yield under short season conditions. Plant Soil 1997, 188, 329–335. [Google Scholar] [CrossRef]
- Hungria, M.; Vargas, M.A. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crop. Res. 2000, 65, 151–164. [Google Scholar] [CrossRef]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–Host Interactions and Symbiotic Nitrogen Fixation in Legume Crops Toward Agriculture Sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef]
- Singla, P.; Garg, N. Plant flavonoids: Key players in signaling, establishment, and regulation of rhizobial and mycorrhizal endosymbioses. In Mycorrhiza-Function, Diversity, State of the Art; Springer: Berlin/Heidelberg, Germany, 2017; pp. 133–176. [Google Scholar]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid mediated selective crosstalk between plants and beneficial soil microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef]
- Massalha, H.; Korenblum, E.; Tholl, D.; Aharoni, A. Small molecules below-ground: The role of specialized metabolites in the rhizosphere. Plant J. 2017, 90, 788–807. [Google Scholar] [CrossRef]
- Lone, R.; Baba, S.H.; Khan, S.; Al-Sadi, A.M.; Kamili, A.N. Phenolics: Key Players in Interaction between Plants and Their Environment. In Plant Phenolics in Abiotic Stress Management (23–46); Springer Nature: Singapore, 2023. [Google Scholar]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar]
- Dong, W.; Song, Y. The Significance of Flavonoids in the Process of Biological Nitrogen Fixation. Int. J. Mol. Sci. 2020, 21, 5926. [Google Scholar] [CrossRef]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere Bacterial Signalling: A Love Parade Beneath Our Feet. Crit. Rev. Microbiol. 2004, 30, 205–240. [Google Scholar] [CrossRef]
- Abd-Alla, M.H. Nodulation and nitrogen fixation in interspecies grafts of soybean and common bean is controlled by iso-flavonoid signal molecules translocated from shoot. Plant Soil Environ. 2011, 57, 453–458. [Google Scholar] [CrossRef]
- Haichar, F.e.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Bashandy, S.R.; Bagy, M.K.; El-Enany, A.-W.E. Rhizobium tibeticum activated with a mixture of flavonoids alleviates nickel toxicity in symbiosis with fenugreek (Trigonella foenum graecum L.). Ecotoxicology 2014, 23, 946–959. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; El-Enany, A.-W.E.; Bagy, M.K.; Bashandy, S.R. Alleviating the inhibitory effect of salinity stress on nod gene expression in Rhizobium tibeticum—fenugreek (Trigonella foenum graecum) symbiosis by isoflavonoids treatment. J. Plant Interact. 2013, 9, 275–284. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Xiao, J.; Tang, L.; Zheng, Y. Interactive influences of intercropping by nitrogen on flavonoid exudation and nodulation in faba bean. Sci. Rep. 2019, 9, 4818. [Google Scholar] [CrossRef] [PubMed]
- Muzika, R.-M. Terpenes and phenolics in response to nitrogen fertilization: A test of the carbon/nutrient balance hypothesis. Chemoecology 1993, 4, 3–7. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Rahmat, A.; Rahman, Z.A. Effects of nitrogen fertilization on synthesis of primary and secondary metabolites in three varieties of kacipertilih (Labisia pumila Blume). Int. J. Mol. Sci. 2011, 12, 5238–5254. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Yamazaki, Y.; Hamamoto, S.; Takase, H.; Yazaki, K. Synthesis and Secretion of Isoflavones by Field-Grown Soybean. Plant Cell Physiol. 2017, 58, 1594–1600. [Google Scholar] [CrossRef]
- Sugiyama, A.; Yamazaki, Y.; Yamashita, K.; Takahashi, S.; Nakayama, T.; Yazaki, K. Developmental and nutritional regulation of isoflavone secretion from soybean roots. Biosci. Biotechnol. Biochem. 2016, 80, 89–94. [Google Scholar] [CrossRef]
- Das, K.; Prasanna, R.; Saxena, A.K. Rhizobia: A potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol. 2017, 62, 425–435. [Google Scholar] [CrossRef]
- Lyu, X.; Sun, C.; Lin, T.; Wang, X.; Li, S.; Zhao, S.; Gong, Z.; Wei, Z.; Yan, C.; Ma, C. Systemic regulation of soybean nodulation and nitrogen fixation by nitrogen via isoflavones. Front. Plant Sci. 2022, 13, 968496. [Google Scholar] [CrossRef]
- Basile, L.A.; Lepek, V.C. Legume–Rhizobium dance: An agricultural tool that could be improved? Microb. Biotechnol. 2021, 14, 1897–1917. [Google Scholar] [CrossRef]
- Abd-Alla, M.H. Regulation of nodule formation in soybean-Bradyrhizobium symbiosis is controlled by shoot or/and root sig-nals. Plant Growth Regul. 2001, 34, 241–250. [Google Scholar] [CrossRef]
- Yokota, K.; Fukai, E.; Madsen, L.H.; Jurkiewicz, A.; Rueda, P.; Radutoiu, S.; Held, M.; Hossain, M.S.; Szczyglowski, K.; Morieri, G.; et al. Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 2009, 21, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, P.; Kereszt, A.; Kondorosi, E. Gene Expression in Nitrogen-Fixing Symbiotic Nodule Cells in Medicago truncatula and Other Nodulating Plants. Plant Cell 2019, 32, 42–68. [Google Scholar] [CrossRef] [PubMed]
- Munoz Aguilar, J.M.; Ashby, A.M.; Richards, A.J.; Loake, G.J.; Watson, M.D.; Shaw, C.H. Chemotaxis of Rhizobium le-guminosarum biovar phaseoli towards flavonoid inducers of the symbiotic nodulation genes. Microbiology 1988, 134, 2741–2746. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots nodule symbiosiss with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef]
- Fournier, J.; Teillet, A.; Chabaud, M.; Ivanov, S.; Genre, A.; Limpens, E.; de Carvalho-Niebel, F.; Barker, D.G. Re-modeling of the infection chamber before infection thread formation reveals a two-step mechanism for rhizobial entry into the host legume root hair. Plant Physiol. 2015, 167, 1233–1242. [Google Scholar] [CrossRef]
- Fournier, J.; Timmers, A.C.; Sieberer, B.J.; Jauneau, A.; Chabaud, M.; Barker, D.G. Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization. Plant Physiol. 2008, 148, 1985–1995. [Google Scholar] [CrossRef]
- Oldroyd, G.E.; Downie, J.A. Coordinating Nodule Morphogenesis with Rhizobial Infection in Legumes. Annu. Rev. Plant Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume–Rhizobium symbioses. J. Integr. Plant Biol. 2021, 64, 244–267. [Google Scholar] [CrossRef]
- Suzaki, T.; Yoro, E.; Kawaguchi, M. Leguminous plants: Inventors of root nodules to accommodate symbiotic bacteria. Int. Rev. Cell Mol. Biol. 2015, 316, 111–158. [Google Scholar]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How rhizobial symbionts invade plants: The Si-norhizobium–Medicago model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef]
- Catoira, R.; Galera, C.; de Billy, F.; Penmetsa, R.V.; Journet, E.P.; Maillet, F.; Rosenberg, C.; Cook, D.; Gough, C.; Dénarié, J. Four genes of Medicago truncatula controlling components of a Nod factor transduction pathway. Plant Cell 2000, 12, 1647–1665. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Rogato, A.; Apuzzo, E.; Omrane, S.; Chiurazzi, M. Differential effects of combined N sources on early steps of the nod factor–dependent transduction pathway in Lotus japonicus. Mol. Plant-Microbe Interact. 2007, 20, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.; Mandal, S. Nod–factors are dispensable for nodulation: A twist in bradyrhizobia-legume symbiosis. Symbiosis 2022, 86, 1–15. [Google Scholar] [CrossRef]
- Calderón-Flores, A.; Du Pont, G.; Huerta-Saquero, A.; Merchant-Larios, H.; Servín-González, L.; Durán, S. The Stringent Response Is Required for Amino Acid and Nitrate Utilization, Nod Factor Regulation, Nodulation, and Nitrogen Fixation in Rhizobium etli. J. Bacteriol. 2005, 187, 5075–5083. [Google Scholar] [CrossRef]
- Okamoto, S.; Ohnishi, E.; Sato, S.; Takahashi, H.; Nakazono, M.; Tabata, S.; Kawaguchi, M. Nod fac-tor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol. 2009, 50, 67–77. [Google Scholar] [CrossRef]
- Endre, G.; Kereszt, A.; Kevei, Z.; Mihacea, S.; Kaló, P.; Kiss, G.B. A receptor kinase gene regulating symbiotic nodule development. Nature 2002, 417, 962–966. [Google Scholar] [CrossRef]
- Nguyen, T.H.N.; Brechenmacher, L.; Aldrich, J.T.; Clauss, T.R.; Gritsenko, M.A.; Hixson, K.K.; Libault, M.; Tanaka, K.; Yang, F.; Yao, Q.; et al. Quantitative phosphoproteomic analysis of soybean root hairs inoculated with Brady-rhizobium japonicum. Mol. Cell. Proteom. 2012, 11, 1140–1155. [Google Scholar] [CrossRef]
- Esseling, J.J.; Lhuissier, F.G.; Emons, A.M.C. A Nonsymbiotic Root Hair Tip Growth Phenotype in NORK-Mutated Legumes: Implications for Nodulation Factor–Induced Signaling and Formation of a Multifaceted Root Hair Pocket for Bacteria. Plant Cell 2004, 16, 933–944. [Google Scholar] [CrossRef]
- Popp, C.; Ott, T. Regulation of signal transduction and bacterial infection during root nodule symbiosis. Curr. Opin. Plant Biol. 2011, 14, 458–467. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; He, Y.; Sang, T.; Wang, P.; Dai, S.; Zhang, S.; Meng, X. Differential phosphorylation of the transcription factor WRKY33 by the protein kinases CPK5/CPK6 and MPK3/MPK6 cooperatively regulates camalexin biosynthesis in Arabidopsis. Plant Cell 2020, 32, 2621–2638. [Google Scholar] [CrossRef]
- Wang, L.; Deng, L.; Bai, X.; Jiao, Y.; Cao, Y.; Wu, Y. Regulation of nodule number by GmNORK is dependent on expression of GmNIC in soybean. Agrofor. Syst. 2019, 94, 221–230. [Google Scholar] [CrossRef]
- Rispail, N.; Kaló, P.; Kiss, G.B.; Ellis, T.N.; Gallardo, K.; Thompson, R.D.; Prats, E.; Larrainzar, E.; Ladrera, R.; González, E.M.; et al. Model legumes contribute to faba bean breeding. Field Crop. Res. 2010, 115, 253–269. [Google Scholar] [CrossRef]
- Keyser, Z.P. Connecting Signaling Mechanisms for Symbiotic Associations: From Mosses to Legumes; The University of Wisconsin-Madison: Madison, WI, USA, 2021. [Google Scholar]
- Bersoult, A.; Camut, S.; Perhald, A.; Kereszt, A.; Kiss, G.B.; Cullimore, J.V. Expression of the Medicago truncatula DMI2 Gene Suggests Roles of the Symbiotic Nodulation Receptor Kinase in Nodules and During Early Nodule Development. Mol. Plant-Microbe Interact. 2005, 18, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Perhald, A.; Endre, G.; Kevei, Z.; Kiss, G.B.; Kereszt, A. Strategies to obtain stable transgenic plants from non-embryogenic lines: Complementation of the nn 1 mutation of the NORK gene in Medicago sativa MN1008. Plant Cell Rep. 2006, 25, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Jeudy, C.; Ruffel, S.; Freixes, S.; Tillard, P.; Santoni, A.L.; Morel, S.; Journet, E.; Duc, G.; Gojon, A.; Lepetit, M.; et al. Adaptation of Medicago truncatula to nitrogen limitation is modulated via local and systemic nodule developmental responses. New Phytol. 2009, 185, 817–828. [Google Scholar] [CrossRef]
- Reid, D.E.; Ferguson, B.J.; Gresshoff, P.M.; Yoro, E.; Suzaki, T.; Kawaguchi, M.; Wang, C.; Yu, H.; Zhang, Z.; Yu, L.; et al. Inoculation- and Nitrate-Induced CLE Peptides of Soybean Control NARK-Dependent Nodule Formation. Mol. Plant-Microbe Interact. 2011, 24, 606–618. [Google Scholar] [CrossRef]
- Oldroyd, G.E.; Downie, J.A. Calcium, kinases and nodulation signaling in legumes. Nat. Rev. Mol. Cell Biol. 2004, 5, 566–576. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Fu, Y.; Shao, J.; Liu, Y.; Xuan, W.; Xu, G.; Zhang, R. Signal communication during microbial modulation of root-system architecture. J. Exp. Bot. 2023, erad263. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Xu, M.; Xiao, Z.; Liu, C.; Du, B.; Xu, D.; Li, L. Signal Molecules Regulate the Synthesis of Secondary Metabolites in the Interaction between Endophytes and Medicinal Plants. Processes 2023, 11, 849. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Svistoonoff, S.; Hocher, V.; Gherbi, H. Actinorhizal root nodule symbioses: What is signalling telling on the origins of nodulation? Curr. Opin. Plant Biol. 2014, 20, 11–18. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2009, 425, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Luo, F.; Gleason, C.; Poovaiah, B.W. Calcium/calmodulin-mediated microbial symbiotic interactions in plants. Front. Plant Sci. 2022, 13, 984909. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 2019, 32, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, M.; Sun, J.; Martins, T.V.; Radhakrishnan, G.V.; Findlay, K.; Soumpourou, E.; Thouin, J.; Véry, A.A.; Sanders, D.; Morris, R.J.; et al. Nuclear-localized cyclic nucleotide–gated channels mediate symbiotic calcium oscillations. Science 2016, 352, 1102–1105. [Google Scholar] [CrossRef]
- Chaulagain, D.; Frugoli, J. The Regulation of Nodule Number in Legumes Is a Balance of Three Signal Transduction Pathways. Int. J. Mol. Sci. 2021, 22, 1117. [Google Scholar] [CrossRef]
- Lebedeva, M.; Azarakhsh, M.; Yashenkova, Y.; Lutova, L. Nitrate-Induced CLE Peptide Systemically Inhibits Nodulation in Medicago truncatula. Plants 2020, 9, 1456. [Google Scholar] [CrossRef]
- Javot, H.; Penmetsa, R.V.; Breuillin, F.; Bhattarai, K.K.; Noar, R.D.; Gomez, S.K.; Zhang, Q.; Cook, D.R.; Harrison, M.J. Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis. Plant J. 2011, 68, 954–965. [Google Scholar] [CrossRef]
- Carbonnel, S.; Gutjahr, C. Control of arbuscular mycorrhiza development by nutrient signals. Front. Plant Sci. 2014, 5, 462. [Google Scholar] [CrossRef]
- Nasrollahi, V.; Allam, G.; Kohalmi, S.E.; Hannoufa, A. MsSPL9 Modulates Nodulation under Nitrate Sufficiency Condition in Medicago sativa. Int. J. Mol. Sci. 2023, 24, 9615. [Google Scholar] [CrossRef]
- de Billy, F.; Grosjean, C.; May, S.; Bennett, M.; Cullimore, J.V. Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol. Plant-Microbe Interact. 2001, 14, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Cho, H.; Choi, D.; Hwang, I. Plant hormonal regulation of nitrogen-fixing nodule organogenesis. Mol. Cells 2012, 34, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Suzuki, A. Effects of phytohormones on nodulation and nitrogen fixation in leguminous plants. In Advances In Biology and Ecology of Nitrogen Fixation; InTech: Rijeka, Croatia, 2014; pp. 111–128. [Google Scholar]
- Gamas, P.; Brault, M.; Jardinaud, M.-F.; Frugier, F. Cytokinins in Symbiotic Nodulation: When, Where, What For? Trends Plant Sci. 2017, 22, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.; Nadzieja, M.; Novák, O.; Heckmann, A.B.; Sandal, N.; Stougaard, J. Cytokinin Biosynthesis Promotes Cortical Cell Responses during Nodule Development. Plant Physiol. 2017, 175, 361–375. [Google Scholar] [CrossRef]
- Mohd-Radzman, N.A.; Djordjevic, M.A.; Imin, N. Nitrogen modulation of legume root architecture signaling pathways involves phytohormones and small regulatory molecules. Front. Plant Sci. 2013, 4, 385. [Google Scholar] [CrossRef]
- Miri, M.; Janakirama, P.; Held, M.; Ross, L.; Szczyglowski, K. Into the Root: How Cytokinin Controls Rhizobial Infection. Trends Plant Sci. 2015, 21, 178–186. [Google Scholar] [CrossRef]
- Reid, D.E.; Heckmann, A.B.; Novák, O.; Kelly, S.; Stougaard, J. Cytokinin Oxidase/Dehydrogenase3 Maintains Cytokinin Homeostasis during Root and Nodule Development in Lotus japonicus. Plant Physiol. 2015, 170, 1060–1074. [Google Scholar] [CrossRef]
- Gupta, R.; Anand, G.; Bar, M. Developmental Phytohormones: Key Players in Host-Microbe Interactions. J. Plant Growth Regul. 2023, 1–22. [Google Scholar] [CrossRef]
- El-Showk, S.; Ruonala, R.; Helariutta, Y. Crossing paths: Cytokinin signalling and crosstalk. Development 2013, 140, 1373–1383. [Google Scholar] [CrossRef]
- Suzaki, T.; Ito, M.; Kawaguchi, M. Genetic basis of cytokinin and auxin functions during root nodule development. Front. Plant Sci. 2013, 4, 42. [Google Scholar] [CrossRef]
- Chan, P.K.; Gresshoff, P.M. Roles of plant hormones in legume nodulation. In Biotechnology-Volume VIII: Funda-Mentals in Biotechnology; EOLSS Publications: Paris, France, 2009; Volume 8, p. 329. [Google Scholar]
- Rafique, M.; Naveed, M.; Mustafa, A.; Akhtar, S.; Munawar, M.; Kaukab, S.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z. The combined effects of gibberellic acid and Rhizobium on growth, yield and nutritional status in chickpea (Cicer arietinum L.). Agronomy 2021, 11, 105. [Google Scholar] [CrossRef]
- Abualia, R.; Riegler, S.; Benkova, E. Nitrate, Auxin and Cytokinin—A Trio to Tango. Cells 2023, 12, 1613. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Roswanjaya, Y.P.; Kohlen, W.; Stougaard, J.; Reid, D. Nitrate restricts nodule organogenesis through inhibition of cytokinin biosynthesis in Lotus japonicus. Nat. Commun. 2021, 12, 6544. [Google Scholar] [CrossRef] [PubMed]
- Caba, J.M.; Centeno, M.L.; Fernández, B.; Gresshoff, P.M.; Ligero, F. Inoculation and nitrate alter phytohormone levels in soybean roots: Differences between a supernodulating mutant and the wild type. Planta 2000, 211, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Jhu, M.Y.; Oldroyd, G.E. Dancing to a different tune, can we switch from chemical to biological nitrogen fixation for sustainable food security? PLoS Biol. 2023, 21, e3001982. [Google Scholar] [CrossRef] [PubMed]
- Becana, M.; Dalton, D.A.; Moran, J.F.; Iturbe-Ormaetxe, I.; Matamoros, M.A.; Rubio, M.C. Reactive oxygen species and antioxidants in legume nodules. Physiol. Plant. 2000, 109, 372–381. [Google Scholar] [CrossRef]
- Pauly, N.; Pucciariello, C.; Mandon, K.; Innocenti, G.; Jamet, A.; Baudouin, E.; Hérouart, D.; Frendo, P.; Puppo, A. Reactive oxygen and nitrogen species and glutathione: Key players in the legume-Rhizobium symbiosis. J. Exp. Bot. 2006, 57, 1769–1776. [Google Scholar] [CrossRef]
- Minchin, F.R.; James, E.K.; Becana, M. Oxygen diffusion, production of reactive oxygen and nitrogen species, and antioxidants in legume nodules. Nitrogen-Fixing Legum. Symbioses 2008, 7, 321–362. [Google Scholar] [CrossRef]
- Nanda, A.K.; Andrio, E.; Marino, D.; Pauly, N.; Dunand, C. Reactive Oxygen Species during Plant-microorganism Early Interactions. J. Integr. Plant Biol. 2010, 52, 195–204. [Google Scholar] [CrossRef]
- Hérouart, D.; Baudouin, E.; Frendo, P.; Harrison, J.; Santos, R.; Jamet, A.; Van de Sype, G.; Touati, D.; Puppo, A. Reactive oxygen species, nitric oxide and glutathione: A key role in the establishment of the legume–Rhizobium symbiosis? Plant Physiol. Biochem. 2002, 40, 619–624. [Google Scholar] [CrossRef]
- Peleg-Grossman, S.; Volpin, H.; Levine, A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J. Exp. Bot. 2007, 58, 1637–1649. [Google Scholar] [CrossRef]
- Lohar, D.P.; Haridas, S.; Gantt, J.S.; VandenBosch, K.A. A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume–rhizobia symbiosis. New Phytol. 2006, 173, 39–49. [Google Scholar] [CrossRef]
- Minguillón, S.; Matamoros, M.A.; Duanmu, D.; Becana, M. Signaling by reactive molecules and antioxidants in legume nodules. New Phytol. 2022, 236, 815–832. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in plants– maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and Development of the Legume-Rhizobial Symbiotic Interface in Infection Threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef]
- Mazars, C.; Thuleau, P.; Lamotte, O.; Bourque, S. Cross-Talk between ROS and Calcium in Regulation of Nuclear Activities. Mol. Plant 2010, 3, 706–718. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant–Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Lecona, A.M.; Nanjareddy, K.; Blanco, L.; Piazza, V.; Vera-Núñez, J.A.; Lara, M.; Arthikala, M.-K. CRK12: A Key Player in Regulating the Phaseolus vulgaris-Rhizobium tropici Symbiotic Interaction. Int. J. Mol. Sci. 2023, 24, 11720. [Google Scholar] [CrossRef]
- Abeed, A.H.; Saleem, M.H.; Asghar, M.A.; Mumtaz, S.; Ameer, A.; Ali, B.; Alwahibi, M.S.; Elshikh, M.S.; Ercisli, S.; El-sharkawy, M.M.; et al. Ameliorative Effects of Exogenous Potassium Nitrate on Antioxidant Defense System and Mineral Nutrient Uptake in Radish (Raphanus sativus L.) under Salinity Stress. ACS Omega 2023, 8, 22575–22588. [Google Scholar] [CrossRef]
- Borella, J.; Becker, R.; Lima, M.C.; Oliveira, D.D.S.C.D.; Braga, E.J.B.; Oliveira, A.C.B.D.; Amarante, L.D. Ni-trogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci. Agric. 2019, 76, 51–62. [Google Scholar] [CrossRef]
- Shah, S.; Chen, C.; Sun, Y.; Wang, D.; Nawaz, T.; El-Kahtany, K.; Fahad, S. Mechanisms of nitric oxide in-volvement in plant-microbe interaction and its enhancement of stress resistance. Plant Stress 2023, 10, 100191. [Google Scholar] [CrossRef]
- Weese, D.J.; Heath, K.D.; Dentinger, B.T.M.; Lau, J.A. Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evolution 2015, 69, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Aroney, S.T.N.; Poole, P.S.; Sánchez-Cañizares, C. Rhizobial Chemotaxis and Motility Systems at Work in the Soil. Front. Plant Sci. 2021, 12, 725338. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Ravi, L. Screening of symbiotic ability of Rhizobium under hydroponic conditions. In Microbial Symbionts; Academic Press: Cambridge, MA, USA, 2023; pp. 327–341. [Google Scholar]
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2019, 13, 1314–1335. [Google Scholar] [CrossRef]
- Oono, R.; Muller, K.E.; Ho, R.; Jimenez Salinas, A.; Denison, R.F. How do less-expensive nitrogen alternatives affect legume sanctions on rhizobia? Ecol. Evol. 2020, 10, 10645–10656. [Google Scholar]
- Burghardt, L.T.; Epstein, B.; Hoge, M.; Trujillo, D.I.; Tiffin, P. Host-Associated Rhizobial Fitness: Dependence on Nitrogen, Density, Community Complexity, and Legume Genotype. Appl. Environ. Microbiol. 2022, 88, e0052622. [Google Scholar] [CrossRef]
- Wendlandt, C.E.; Gano-Cohen, K.A.; Stokes, P.J.N.; Jonnala, B.N.R.; Zomorrodian, A.J.; Al-Moussawi, K.; Sachs, J.L. Wild legumes maintain beneficial soil rhizobia populations despite decades of nitrogen deposition. Oecologia 2022, 198, 419–430. [Google Scholar] [CrossRef]
- Godschalx, A.L.; Diethelm, A.C.; Kautz, S.; Ballhorn, D.J. Nitrogen-Fixing Rhizobia Affect Multitrophic Interactions in the Field. J. Insect Behav. 2023, 36, 168–179. [Google Scholar] [CrossRef]
- Brito-Santana, P.; Duque-Pedraza, J.J.; Bernabéu-Roda, L.M.; Carvia-Hermoso, C.; Cuéllar, V.; Fuentes-Romero, F.; Acosta-Jurado, S.; Vinardell, J.M.; Soto, M.J. Sinorhizobium meliloti DnaJ Is Required for Surface Motility, Stress Tolerance, and for Efficient Nodulation and Symbiotic Nitrogen Fixation. Int. J. Mol. Sci. 2023, 24, 5848. [Google Scholar]
- Ohyama, T.; Ikebe, K.; Okuoka, S.; Ozawa, T.; Nishiura, T.; Ishiwata, T.; Yamazaki, A.; Tanaka, F.; Takahashi, T.; Umezawa, T.; et al. A deep placement of lime nitrogen reduces the nitrate leaching and promotes soybean growth and seed yield. Crop. Environ. 2022, 1, 221–230. [Google Scholar] [CrossRef]
- Ohyama, T.; Takayama, K.; Akagi, A.; Saito, A.; Higuchi, K.; Sato, T. Development of an N-Free Culture Solution for Cultivation of Nodulated Soybean with Less pH Fluctuation by the Addition of Potassium Bicarbonate. Agriculture 2023, 13, 739. [Google Scholar] [CrossRef]
- Tambalo, D.D.; Yost, C.K.; Hynes, M.F. Motility and chemotaxis in the rhizobia. In Biological Nitrogen Fixation; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 337–348. [Google Scholar]
- Raina, J.-B.; Fernandez, V.; Lambert, B.; Stocker, R.; Seymour, J.R. The role of microbial motility and chemotaxis in symbiosis. Nat. Rev. Microbiol. 2019, 17, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.K.; Brown, M.R.; Subramanian, S.; Brözel, V.S. Bradyrhizobium diazoefficiens USDA 110 displays plasticity in the attachment phenotype when grown in different soybean root exudate compounds. Front. Microbiol. 2023, 14, 1190396. [Google Scholar] [CrossRef]
- Tham, I.; Tham, F.Y. Effects of nitrogen on nodulation and promiscuity in the Acacia mangium rhizobia relationship. Asian J. Plant Sci. 2007, 6, 941–948. [Google Scholar] [CrossRef]
- Ohyama, T.; Fujikake, H.; Yashima, H.; Tanabata, S.; Ishikawa, S.; Sato, T.; Nishiwaki, T.; Ohtake, N.; Sueyoshi, K.; Ishii, S.; et al. Effect of nitrate on nodulation and nitrogen fixation of soybean. Soybean Physiol. Biochem. 2011, 10, 333–364. [Google Scholar]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of Nitrate on Nodule and Root Growth of Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef]
- Herliana, O.; Harjoso, T.; Anwar, A.H.S.; Fauzi, A. The effect of Rhizobium and N fertilizer on growth and yield of black soybean (Glycine max (L) Merril). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 255, p. 012015. [Google Scholar]
- Garg, N.; Geetanjali. Symbiotic nitrogen fixation in legume nodules: Process and signaling: A review. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 519–531. [Google Scholar]
- Mendoza-Suárez, M.; Andersen, S.U.; Poole, P.S.; Sánchez-Cañizares, C. Competition, Nodule Occupancy, and Persistence of Inoculant Strains: Key Factors in the Rhizobium-Legume Symbioses. Front. Plant Sci. 2021, 12, 690567. [Google Scholar] [CrossRef]
- Murray, J.D. Invasion by Invitation: Rhizobial Infection in Legumes. Mol. Plant-Microbe Interact. 2011, 24, 631–639. [Google Scholar] [CrossRef]
- Nishida, H.; Suzaki, T. Nitrate-mediated control of root nodule symbiosis. Curr. Opin. Plant Biol. 2018, 44, 129–136. [Google Scholar] [CrossRef]
- Ralston, E.J.; Imsande, J. Nodulation of hydroponically grown soybean plants and inhibition of nodule development by nitrate. J. Exp. Bot. 1983, 34, 1371–1378. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.-H.; Lin, Y.-H.; Reid, D.E.; Gresshoff, P.M. Molecular Analysis of Legume Nodule Development and Autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Akter, Z.; Lupwayi, N.Z.; Balasubramanian, P. Nitrogen use efficiency of irrigated dry bean (Phaseolus vulgaris L.) genotypes in southern Alberta. Can. J. Plant Sci. 2017, 97, 610–619. [Google Scholar] [CrossRef]
- Akter, Z.; Pageni, B.B.; Lupwayi, N.Z.; Balasubramanian, P.M. Biological nitrogen fixation by irrigated dry bean (Phaseolus vulgaris L.) genotypes. Can. J. Plant Sci. 2018, 98, 1159–1167. [Google Scholar]
- Reinprecht, Y.; Schram, L.; Marsolais, F.; Smith, T.H.; Hill, B.; Pauls, K.P. Effects of Nitrogen Application on Nitrogen Fixation in Common Bean Production. Front. Plant Sci. 2020, 11, 534817. [Google Scholar] [CrossRef]
- Karmarkar, V. Transcriptional Regulation of Nodule Development and Senescence in Medicago Truncatula; Wageningen University and Research: Wageningen, The Netherlands, 2014. [Google Scholar]
- Farid, M.; Earl, H.J.; Navabi, A. Yield Stability of Dry Bean Genotypes across Nitrogen-Fixation-Dependent and Fertilizer-Dependent Management Systems. Crop. Sci. 2016, 56, 173–182. [Google Scholar] [CrossRef]
- Franck, S.; Strodtman, K.N.; Qiu, J.; Emerich, D.W. Transcriptomic Characterization of Bradyrhizobium diaz-oefficiens Bacteroids Reveals a Post-Symbiotic, Hemibiotrophic-Like Lifestyle of the Bacteria within Senescing Soybean Nodules. Int. J. Mol. Sci. 2018, 19, 3918. [Google Scholar] [CrossRef]
- Strodtman, K.N.; Frank, S.; Stevenson, S.; Thelen, J.J.; Emerich, D.W. Proteomic characterization of Bradyrhi-zobium diazoefficiens bacteroids reveals a post-symbiotic, hemibiotrophic-like lifestyle of the bacteria within senescing soybean nodules. Int. J. Mol. Sci. 2018, 19, 3947. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, C.; Huang, Y.; Chen, H.; Yuan, S.; Zhou, X. Characteristics and Research Progress of Legume Nodule Senescence. Plants 2021, 10, 1103. [Google Scholar] [CrossRef]
- Ono, Y.; Fukasawa, M.; Sueyoshi, K.; Ohtake, N.; Sato, T.; Tanabata, S.; Toyota, R.; Higuchi, K.; Saito, A.; Ohyama, T. Application of nitrate, ammonium, or urea changes the concentrations of ureides, urea, amino acids and other metabolites in xylem sap and in the organs of soybean plants (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2021, 22, 4573. [Google Scholar]
- Li, S.; Wu, C.; Liu, H.; Lyu, X.; Xiao, F.; Zhao, S.; Ma, C.; Yan, C.; Liu, Z.; Li, H.; et al. Systemic regulation of nodule structure and assimilated carbon distribution by nitrate in soybean. Front. Plant Sci. 2023, 14, 1101074. [Google Scholar] [CrossRef]
- Martensson, A.M.; Brutti, L.; Ljunggren, H. Competition between strains of Bradyrhizobium japonicum for nod-ulation of soybeans at different nitrogen fertilizer levels. Plant Soil 1989, 117, 219–225. [Google Scholar] [CrossRef]
- Jiang, Y.; MacLean, D.E.; Perry, G.E.; Marsolais, F.; Hill, B.; Pauls, K.P. Evaluation of beneficial and inhibitory effects of nitrate on nodulation and nitrogen fixation in common bean (Phaseolus vulgaris). Legum. Sci. 2020, 2, e45. [Google Scholar] [CrossRef]
- Dazzo, F.B.; Hrabak, E.M.; Urbano, M.R.; Sherwood, J.E.; Truchet, G. Regulation of recognition in the Rhizobium-clover symbiosis. In Current Perspectives in Nitrogen Fixation; Gibson, A.H., Newton, W.E., Eds.; Australian Academy of Science: Canberra, Australia, 1981; pp. 292–295. [Google Scholar]
- Sherwood, J.E.; Truchet, G.L.; Dazzo, F.B. Effect of nitrate supply on the in-vivo synthesis and distribution ofertilizn A, a Rhizobium trifolii-binding lectin, in Trifolium repens seedlings. Planta 1984, 162, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.H.; Harper, J.E. Nitrate effect on nodulation of soybean by Bradyrhizobium japonicum 1. Crop Sci. 1985, 25, 497–501. [Google Scholar] [CrossRef]
- Kwon, D.; Beevers, H. Adverse effects of nitrate on stem nodules of Sesbania rostrata Brem*. New Phytol. 1993, 125, 345–350. [Google Scholar] [CrossRef]
- Latimore, M., Jr.; Giddens, J.; Ashley, D.A. Effect of Ammonium and Nitrate Nitrogen upon Photosynthate Supply and Nitrogen Fixation by Soybeans 1. Crop Sci. 1977, 17, 399–404. [Google Scholar] [CrossRef]
- Wong, P.P. Nitrate and Carbohydrate Effects on Nodulation and Nitrogen Fixation (Acetylene Reduction) Activity of Lentil (Lens esculenta Moench). Plant Physiol. 1980, 66, 78–81. [Google Scholar] [CrossRef]
- Khan, A.A.; Khan, A.A. Effects of nitrate nitrogen on growth, nodulation and distribution of 14 C-labelled photosynthates in cowpea. Plant Soil 1981, 63, 141–147. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Tsai, S.M.; Bonetti, R. Response to inoculation and N fertilization for increased yield and biological nitrogen fixation of common bean (Phaseolus vulgaris L.). Enhancement of Biological Nitrogen Fixation of Common Bean in Latin America: Results from an FAO/IAEA Coordinated Research Programme. Plant Soil 1993, 152, 123–130. [Google Scholar] [CrossRef]
- Afza, R.; Hardarson, G.; Zapata, F.; Danso, S.K.A. Effects of delayed soil and foliar N fertilization on yield and N2 fixation of soybean. Plant Soil 1987, 97, 361–368. [Google Scholar] [CrossRef]
- Wolyn, D.J.; Attewell, J.; Ludden, P.W.; Bliss, F.A. Indirect measures of N 2 fixation in common bean (Phaseolus vulgaris L.) under field conditions: The role of lateral root nodules. Plant Soil 1989, 113, 181–187. [Google Scholar] [CrossRef]
- Cheniae, G.; Evans, H.J. Physiological Studies on Nodule-Nitrate Reductase. Plant Physiol. 1960, 35, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.H.; Pagan, J.D. Nitrate effects on the nodulation of legumes inoculated with nitrate-reductase-deficient mutants of Rhizobium. Planta 1977, 134, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Manhart, J.R.; Wong, P.P. Nitrate effect on nitrogen fixation (acetylene reduction) activities of legume root nodules induced by rhizobia with varied nitrate reductase activities. Plant Physiol. 1980, 65, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.G. Synthesis and Accumulation of Nitrite in Soybean Nodules Supplied with Nitrate. Plant Physiol. 1982, 69, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.D.; Neyra, C.A. Nitrate and Nitrite Reduction in Relation to Nitrogenase Activity in Soybean Nodules and Rhizobium japonicum Bacteroids. Plant Physiol. 1983, 71, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S.; Kumar, S.; Dhillon, K.S.; Singh, R. Effect of Nitrogen on Nitrate Reductase Activity in the Nodules and Leaves of Summer Moong (Vigna radiata). Ann. Bot. 1986, 58, 515–521. [Google Scholar] [CrossRef]
- Minchin, F.R.; Becana, M.; Sprent, J.I. Short-term inhibition of legume N 2 fixation by nitrate: II. Nitrate effects on nodule oxygen diffusion. Planta 1989, 180, 46–52. [Google Scholar] [CrossRef]
- Becana, M.; Minchin, F.R.; Sprent, J.I. Short-term inhibition of legume N 2 fixation by nitrate: I. Nitrate effects on nitrate-reductase activities of bacteroids and nodule cytosol. Planta 1989, 180, 40–45. [Google Scholar] [CrossRef]
- Meakin, G.E.; Bueno, E.; Jepson, B.; Bedmar, E.J.; Richardson, D.J.; Delgado, M.J. The contribution of bacteroidal nitrate and nitrite reduction to the formation of nitrosylleghaemoglobin complexes in soybean root nodules. Microbiology 2007, 153, 411–419. [Google Scholar] [CrossRef]
- Sánchez, C.; Gates, A.J.; Meakin, G.E.; Uchiumi, T.; Girard, L.; Richardson, D.J.; Bedmar, E.J.; Delgado, M.J. Production of Nitric Oxide and Nitrosylleghemoglobin Complexes in Soybean Nodules in Response to Flooding. Mol. Plant-Microbe Interact. 2010, 23, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, Y.; Yamamoto, Y. Inhibition of Nitrogen Fixation in Soybean Plants Supplied with Nitrate II. Accumul. Prop. Nitrosylleghemoglobin Nodules. Plant Cell Physiol. 1990, 31, 207–214. [Google Scholar] [CrossRef]
- Kanayama, Y.; Yamamoto, Y. Inhibition of nitrogen fixation in soybean plants supplied with nitrate III. Kinetics of the formation of nitrosylleghaemoglobin and of the inhibition of formation of oxyleghaemoglobin. Plant Cell Physiol. 1990, 31, 603–608. [Google Scholar]
- Kanayama, Y.; Watanabe, I.; Yamamoto, Y. Inhibition of nitrogen fixation in soybean plants supplied with nitrate I. Nitrite accumulation and formation of nitrosylleghaemoglobin in nodules. Plant Cell Physiol. 1990, 31, 341–346. [Google Scholar]
- Horchani, F.; Prévot, M.; Boscari, A.; Evangelisti, E.; Meilhoc, E.; Bruand, C.; Raymond, P.; Boncompagni, E.; Aschi-Smiti, S.; Puppo, A.; et al. Both Plant and Bacterial Nitrate Reductases Contribute to Nitric Oxide Production in Medicago truncatula Nitrogen-Fixing Nodules. Plant Physiol. 2010, 155, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Cam, Y.; Pierre, O.; Boncompagni, E.; Hérouart, D.; Meilhoc, E.; Bruand, C. Nitric oxide (NO): A key player in the senescence of Medicago truncatula root nodules. New Phytol. 2012, 196, 548–560. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Horta Araujo, N.; Maucourt, M.; Hanchi, M.; Bernillon, S.; Rolin, D.; Puppo, A.; Brouquisse, R. Plant nitrate reductases regulate nitric oxide production and nitrogen-fixing metabolism during the Medicago trun-catula–Sinorhizobium meliloti symbiosis. Front. Plant Sci. 2020, 11, 535004. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Puppo, A.; Brouquisse, R. Nitrate reductases and hemoglobins control nitrogen-fixing symbiosis by regulating nitric oxide accumulation. J. Exp. Bot. 2020, 72, 873–884. [Google Scholar] [CrossRef]
- Kubo, H. Uber hamoprotein aus den wurzelknollchen von leguminosen. Acta Phytochimica 1939, 11, 195–200. [Google Scholar]
- Keilin, D.; Wang, Y.L. Haemoglobin of Gastrophilus larvae. Purification and properties. Biochem. J. 1946, 40, 855. [Google Scholar] [CrossRef]
- Virtanen, A.I.; Laine, T. Red, Brown and Green Pigments in Leguminous Root Nodules. Nature 1946, 157, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, J.B.; Bergersen, F.J.; Appleby, C.A.; Turner, G.L. Facilitated oxygen diffusion: The role of leghemo-globin in nitrogen fixation by bacteroids isolated from soybean root nodules. J. Biol. Chem. 1974, 249, 4057–4066. [Google Scholar] [CrossRef] [PubMed]
- Appleby, C.A. The origin and functions of haemoglobin in plants. Sci. Prog. 1933, 76, 365–398. [Google Scholar]
- Appleby, C.A.; Tjepkema, J.D.; Trinick, M.J. Hemoglobin in a non-leguminous plant, Parasponia: Possible genetic origin and function in nitrogen fixation. Science 1983, 220, 951–953. [Google Scholar] [CrossRef]
- Silvester, W.B.; Berg, R.H.; Schwintzer, C.R.; Tjepkema, J.D. Oxygen responses, hemoglobin, and the structure and function of vesicles. In Nitrogen-Fixing Actinorhizal Symbioses; Springer: Dordrecht, The Netherlands, 2008; pp. 105–146. [Google Scholar]
- Becana, M.; Sprent, J.I. Effect of Nitrate on Components of Nodule Leghaemoglobins. J. Exp. Bot. 1989, 40, 725–731. [Google Scholar] [CrossRef]
- Larrainzar, E.; Villar, I.; Rubio, M.C.; Pérez-Rontomé, C.; Huertas, R.; Sato, S.; Mun, J.H.; Becana, M. Hemo-globins in the legume–Rhizobium symbiosis. New Phytol. 2020, 228, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Becana, M.; Yruela, I.; Sarath, G.; Catalán, P.; Hargrove, M.S. Plant hemoglobins: A journey from unicellular green algae to vascular plants. New Phytol. 2020, 227, 1618–1635. [Google Scholar] [CrossRef]
- Fuchsman, W.H.; Appleby, C.A. Separation and determination of the relative concentrations of the homoge-neous components of soybean leghemoglobin by isoelectric focusing. Biochim. Biophys. Acta (BBA)-Protein Struct. 1979, 579, 314–324. [Google Scholar] [CrossRef]
- Uheda, E.; Syōno, K. Physiological Role of Leghaemoglobin Heterogeneity in Pea Root Nodule Development. Plant Cell Physiol. 1982, 23, 75–84. [Google Scholar] [CrossRef]
- Kawashima, K.; Suganuma, N.; Tamaoki, M.; Kouchi, H. Two Types of Pea Leghemoglobin Genes Showing Different O2-Binding Affinities and Distinct Patterns of Spatial Expression in Nodules. Plant Physiol. 2001, 125, 641–651. [Google Scholar] [CrossRef]
- Miller, L.D.; Yost, C.K.; Hynes, M.F.; Alexandre, G. The major chemotaxis gene cluster of Rhizobium legumi-nosarum bv. viciae is essential for competitive nodulation. Mol. Microbiol. 2007, 63, 348–362. [Google Scholar] [PubMed]
- Navascués, J.; Pérez-Rontomé, C.; Gay, M.; Marcos, M.; Yang, F.; Walker, F.A.; Desbois, A.; Abián, J.; Becana, M. Leghemoglobin green derivatives with nitrated hemes evidence production of highly reactive nitrogen species during aging of legume nodules. Proc. Natl. Acad. Sci. USA 2012, 109, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Gao, Z.; Li, X.; Liao, H. Excess nitrate induces nodule greening and reduces transcript and protein expression levels of soybean leghaemoglobins. Ann. Bot. 2020, 126, 61–72. [Google Scholar] [CrossRef]
- Rutledge, H.L.; Cook, B.D.; Nguyen, H.P.; Herzik, M.A., Jr.; Tezcan, F.A. Structures of the nitrogenase complex prepared under catalytic turnover conditions. Science 2022, 377, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.P.; Brill, W.J. Genetics and Regulation of Nitrogen Fixation. Annu. Rev. Microbiol. 1981, 35, 207–235. [Google Scholar] [CrossRef] [PubMed]
- Bisseling, T.; Bos, R.V.D.; Van Kammen, A. The effect of ammonium nitrate on the synthesis of nitrogenase and the concentration of leghemoglobin in pea root nodules induced by Rhizobium leguminosarum. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1978, 539, 1–11. [Google Scholar] [CrossRef]
- Rigaud, J.; Puppo, A. Effect of nitrite upon leghemoglobin and interaction with nitrogen fixation. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1977, 497, 702–706. [Google Scholar] [CrossRef]
- Trinchant, J.C.; Rigaud, J. Nitrogen fixation in French-beans in the presence of nitrate: Effect on bacteroid res-piration and comparison with nitrite. J. Plant Physiol. 1984, 116, 209–217. [Google Scholar] [CrossRef]
- Schuller, K.A.; Day, D.A.; Gibson, A.H.; Gresshoff, P.M. Enzymes of ammonia assimilation and ureide biosynthesis in soybean nodules: Effect of nitrate. Plant Physiol. 1986, 80, 646–650. [Google Scholar] [CrossRef]
- Schuller, K.A.; Minchin, F.R.; Gresshoff, P.M. Nitrogenase Activity and Oxygen Diffusion in Nodules of Soyabean cv. Bragg and a Supernodulating Mutant: Effects of Nitrate. J. Exp. Bot. 1988, 39, 865–877. [Google Scholar] [CrossRef]
- Vessey, J.K.; Walsh, K.B.; Layzell, D.B. Oxygen limitation of N2 fixation in stem-girdled and nitrate-treated soybean. Physiol. Plant. 1988, 73, 113–121. [Google Scholar] [CrossRef]
- Vessey, J.K.; Walsh, K.B.; Layzell, D.B. Can a limitation in phloem supply to nodules account for the inhibitory effect of nitrate on nitrogenase activity in soybean? Physiol. Plant. 1988, 74, 137–146. [Google Scholar] [CrossRef]
- Vessey, J.K.; Waterer, J. In search of the mechanism of nitrate inhibition of nitrogenase activity in legume nodules: Recent developments. Physiol. Plant. 1992, 84, 171–176. [Google Scholar] [CrossRef]
- Layzell, D.B.; Hunt, S.; Palmer, G.R. Mechanism of nitrogenase inhibition in soybean nodules: Pulse-modulated spectroscopy indicates that nitrogenase activity is limited by O2. Plant Physiol. 1990, 92, 1101–1107. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Harper, J.E. The feedback mechanism of nitrate inhibition of nitrogenase activity in soybean may involve asparagine and/or products of its metabolism. Physiol. Plant. 1997, 100, 371–377. [Google Scholar] [CrossRef]
- Berger, A.; Boscari, A.; Frendo, P.; Brouquisse, R. Nitric oxide signaling, metabolism and toxicity in nitro-gen-fixing symbiosis. J. Exp. Bot. 2019, 70, 4505–4520. [Google Scholar] [CrossRef]
- Sasakura, F.; Uchiumi, T.; Shimoda, Y.; Suzuki, A.; Takenouchi, K.; Higashi, S.; Abe, M. A Class 1 Hemoglobin Gene from Alnus firma Functions in Symbiotic and Nonsymbiotic Tissues to Detoxify Nitric Oxide. Mol. Plant-Microbe Interact. 2006, 19, 441–450. [Google Scholar] [CrossRef]
- Kato, K.; Kanahama, K.; Kanayama, Y. Involvement of nitric oxide in the inhibition of nitrogenase activity by nitrate in Lotus root nodules. J. Plant Physiol. 2010, 167, 238–241. [Google Scholar] [CrossRef]
- Compant, S.; Van Der Heijden, M.G.; Sessitsch, A. Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef]
- Olivares, J.; Bedmar, E.J.; Sanjuán, J.; Karpinets, T.V.; Park, B.H.; Syed, M.H.; Klotz, M.G.; Uberbacher, E.C. Biological Nitrogen Fixation in the Context of Global Change. Mol. Plant-Microbe Interact. 2013, 26, 486–494. [Google Scholar] [CrossRef]
- Mabrouk, Y.; Hemissi, I.; Salem, I.B.; Mejri, S.; Saidi, M.; Belhadj, O. Potential of rhizobia in improving nitrogen fixation and yields of legumes. Symbiosis 2018, 107, 1–16. [Google Scholar]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; JR Alves, B.; Morrison, M.J. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Grover, M.; Yaadesh, S.; Jayasurya, A. Associative Nitrogen Fixers-Options for Mitigating Climate Change. In Bioinoculants: Biological Option for Mitigating Global Climate Change (217–237); Springer Nature: Singapore, 2023. [Google Scholar]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.; McCarthy, H.; Hendrey, G.; McNulty, S.G.; et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Kane, D. Carbon Sequestration Potential on Agricultural Lands: A Review of Current Science and Available Practices; National Sustainable Agriculture Coalition Breakthrough Strategies and Solutions, LLC: Memphis, TN, USA, 2015; pp. 1–35. [Google Scholar]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant Nutrition under Climate Change and Soil Carbon Sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Mukhtar, H.; Wunderlich, R.F.; Muzaffar, A.; Ansari, A.; Shipin, O.V.; Cao, T.N.-D.; Lin, Y.-P. Soil microbiome feedback to climate change and options for mitigation. Sci. Total Environ. 2023, 882, 163412. [Google Scholar] [CrossRef]
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Bayu, T. Review on contribution of integrated soil fertility management for climate change mitigation and agri-cultural sustainability. Cogent Environ. Sci. 2020, 6, 1823631. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Jena, J.; Maitra, S.; Hossain, A.; Pramanick, B.; Gitari, H.I.; Praharaj, S.; Shankar, T.; Palai, J.B.; Rathore, A.; Mandal, T.K.; et al. Role of Legumes in Cropping System for Soil Ecosystem Improvement. Ecosystem Services: Types, Management and Benefits; Nova Science Publishers, Inc.: New York, NY, USA, 2022; p. 415. [Google Scholar]
- Muhie, S.H. Novel approaches and practices to sustainable agriculture. J. Agric. Food Res. 2022, 10, 100446. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Rajanna, G.A.; Kumar, A. Integrated Nutrient Management: An Integral Component of ICM Approach. In Integrated Crop Management Practices; ICAR: New Delhi, India, 2018; p. 33. [Google Scholar]
- Gurjar, R.; Tomar, D.; Singh, A.; Kumar, K. Integrated nutrient management and its effect on mungbean (Vigna radiata L. Wilczek): A revisit. Pharma Innov. J. 2022, 11, 379–384. [Google Scholar]
- Tomar, D.; Bhatnagar, G.S. A review on integrated nutrient management and its effect on mung bean (Vigna radiata L. Wilczek). Pharma Innov. J. 2022, 11, 685–691. [Google Scholar]
- Obando, M.; Correa-Galeote, D.; Castellano-Hinojosa, A.; Gualpa, J.; Hidalgo, A.; Alché, J.D.D.; Bedmar, E.; Cassán, F. Analysis of the denitrification pathway and greenhouse gases emissions in Bradyrhizobium sp. strains used as biofertilizers in South America. J. Appl. Microbiol. 2019, 127, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Wong, W.S.; Morald, T.K.; Whiteley, A.S.; Nevill, P.G.; Trengove, R.D.; Yong, J.W.H.; Dixon, K.W.; Valliere, J.M.; Stevens, J.C.; Veneklaas, E.J. Microbial inoculation to improve plant performance in mine-waste substrates: A test using pigeon pea (Cajanus cajan). Land Degrad. Dev. 2021, 33, 497–511. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Mora, C.; Strauss, S.L. Native Rhizobia Improve Plant Growth, Fix N2, and Reduce Greenhouse Emissions of Sunnhemp More than Commercial Rhizobia Inoculants in Florida Citrus Orchards. Plants 2022, 11, 3011. [Google Scholar] [CrossRef]
- Woliy, K.; Degefu, T.; Frostegård, Å. Host Range and Symbiotic Effectiveness of N2O Reducing Bradyrhizobium Strains. Front. Microbiol. 2019, 10, 2746. [Google Scholar] [CrossRef]
- Sharma, V.; Bhattacharyya, S.; Kumar, R.; Kumar, A.; Ibañez, F.; Wang, J.; Guo, B.; Sudini, H.K.; Gopalakrishnan, S.; Dasgupta, M.; et al. Molecular Basis of Root Nodule Symbiosis between Bradyrhizobium and ‘Crack-Entry’ Legume Groundnut (Arachis hypogaea L.). Plants 2020, 9, 276. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.-W.E. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture13112092
Abd-Alla MH, Al-Amri SM, El-Enany A-WE. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture. 2023; 13(11):2092. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture13112092
Chicago/Turabian StyleAbd-Alla, Mohamed Hemida, Salem M. Al-Amri, and Abdel-Wahab Elsadek El-Enany. 2023. "Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change" Agriculture 13, no. 11: 2092. https://0-doi-org.brum.beds.ac.uk/10.3390/agriculture13112092